Soft Tissue Reconstruction of Complete Circumferential Defects of the Upper Extremity
Article information
Abstract
Background
Upper extremity soft tissue defects with complete circumferential involvement are not common. Coupled with the unique anatomy of the upper extremity, the underlying etiology of such circumferential soft tissue defects represent additional reconstructive challenges that require treatment to be tailored to both the patient and the wound. The aim of this study is to review the various options for soft tissue reconstruction of complete circumferential defects in the upper extremity.
Methods
A literature review of PubMed and MEDLINE up to December 2016 was performed. The current study focuses on forearm and arm defects from the level at or proximal to the wrist and were assessed based on Tajima's classification (J Trauma 1974). Data reviewed for analysis included patient demographics, causality, defect size, reconstructive technique(s) employed, and postoperative follow-up and functional outcomes (when available).
Results
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, 14 unique articles were identified for a total of 50 patients (mean=28.1 years). Underlying etiologies varied from extensive thermal or electrical burns to high impact trauma leading to degloving or avulsion, crush injuries, or even occur iatrogenically after tumor extirpation or extensive debridement. Treatment options ranged from the application of negative pressure wound dressings to the opposite end of the spectrum in hand transplantation.
Conclusions
With the evolution of reconstructive techniques over time, the extent of functional and aesthetic rehabilitation of these complex upper extremity injuries has also improved. The proposed management algorithm comprehensively addresses the inherent challenges associated with these complex cases.
INTRODUCTION
The upper extremity is functionally and anatomically complex. Its role in our daily interactions with the external environment translates to an increased susceptibility to various forms of trauma including burns (thermal, electrical), degloving or avulsion and crush injuries of the skin envelope. Occasionally, the resultant soft tissue defect may involve complete circumferential destruction of the overlying skin. Such defects may also result iatrogenically following oncological resection (e.g., neoplastic processes, congenital nevi) and surgical debridement of necrotic tissues (e.g., due to extensive infection). In severe cases, the underlying bony and tendinous structures may be involved as well. These complete circumferential defects are “three-dimensional” in nature with concomitant involvement of the radial, ulnar, volar and dorsal surfaces of the affected limb. In addition to defect size, further considerations such as volume, mobility and pliability are necessary for adequate soft tissue coverage and functional outcomes. Moreover, the nature of the original insult would often preclude the use of local reconstructive options due to proximity to the zone of injury. Treatment options can therefore be limited and represent a particular challenge.
Various grading schemes of these circumferential injuries have been reported previously. Tajima described a classification system for degloving or avulsion and heat-press injuries with increasing depths of anatomic involvement from the deep fascia to periosteum and bone which could then be addressed with skin grafting and/or additional flap transfers respectively [1]. Waikakul [2] then described a system with increasing severity of involvement and introduced revascularization of the arterial and venous systems. More recently, Lo et al. [3] introduced a classification scheme that builds on Waikakul's by designating anatomical areas of involvement distal and proximal to the wrist that are considered “non-expendable” and “expendable” respectively due to involvement, or not, of glabrous skin of the palm and digits.
In recent times, various wound care adjuncts such as negative pressure wound therapy (NPWT) and skin substitutes have received greater recognition because evidence suggests that their use in delayed reconstruction did not affect overall outcomes [4] and in fact, may even simplify the final reconstruction required [5]. In contrast, the use of tissue expansion techniques can further optimize the final aesthetic and functional outcomes in an elective setting. Therefore, the management of complete circumferential injuries of the upper extremity requires further clarification due to the multitude of underlying etiologies and is the aim of this review.
METHODS
Our study focuses on forearm and arm defects from the level at and proximal to the wrist (similar to Group 3 defects described by Lo et al. [3]). A literature review up to December 2016 was performed using PubMed and MEDLINE. Search terms included “upper extremity degloving”, “forearm degloving”, “upper extremity circumferential”, “upper extremity circumferential defect”, “extremity circumferential defect”, “forearm circumferential defect” and “wrist circumferential.” All English language articles were screened and abstracts, when provided, were individually reviewed for relevance to upper extremity soft tissue defects with complete circumferential involvement. Relevant data extracted include patient demographics, causality, defect size, reconstructive technique(s) employed, and postoperative follow-up and functional outcomes. We then applied Tajima's classification [1] to categorize the extent of soft tissue involvement described.
Studies were excluded if (1) the circumferential defect(s) involved only a single digit (including the thumb) or multiple digits not in-continuity because such problems are considered a separate topic of its own, (2) the level of injury was distal to the wrist and salvage attempts are based more on arterio-venous shunting [23] of the degloved or avulsed skin segment, and (3) if there was inadequate data to determine the exact number or extent of cases with complete circumferential involvement. From here, all complete circumferential soft tissue defects of the upper extremity will be referred to simply as “circumferential”.
RESULTS
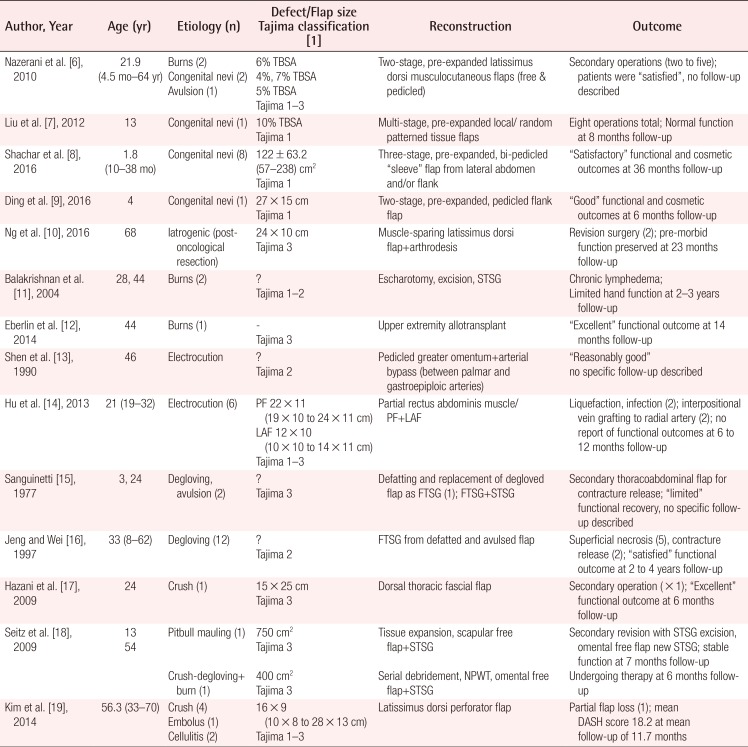
A total of 586 articles were identified based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. After removing duplicate entries and applying our inclusion and exclusion criteria, 14 unique articles were included in the final analysis (Table 1) [678910111213141516171819].
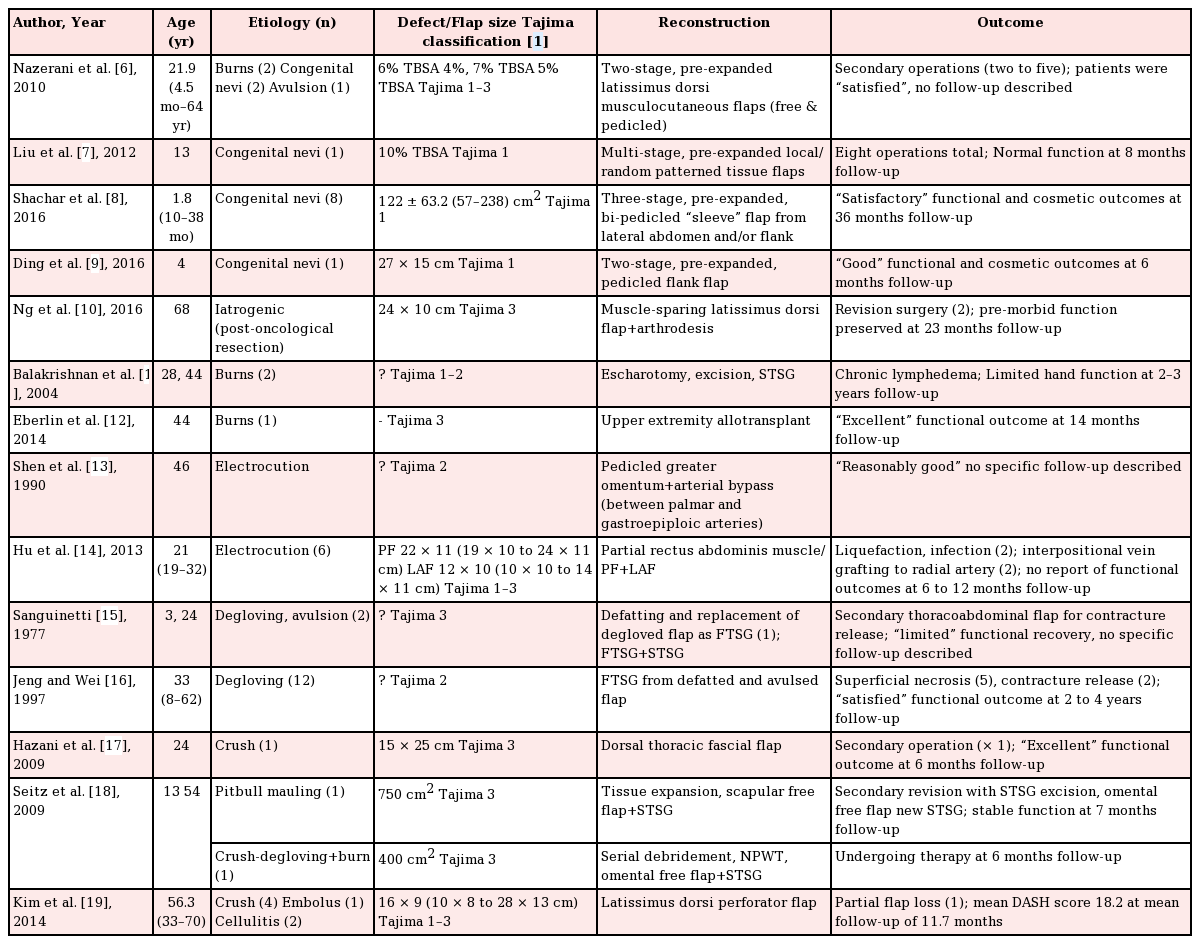
Summary of literature review on soft tissue reconstruction of upper extremity circumferential defects
Overall, 50 patients with a mean age of 28.1 years (range, 4.5 months–70 years) were identified. Trauma (i.e., burns, avulsion, degloving, crush injuries) was the most common cause (68%) of circumferential defects followed by iatrogenic causes (e.g. resections for congenital nevi or neoplasia; extensive debridement). The extent of circumferential defect reported varied from Tajima 1 (superficial to deep fascia), 2 (superficial to periosteum), and 3 (bone affected) with various reconstructive techniques employed including varying combinations of splitthickness skin grafts (STSGs), full-thickness skin grafts (FTSGs), tissue expanded flaps (random, pedicled, free), various flap options (greater omentum, paraumbilical, rectus abdominis, lower abdominal, thoracoabdominal, latissimus dorsi etc.) and even composite tissue allotransplantation.
For congenital nevi with circumferential involvement of the upper extremity, tissue expansion of local and/or regional options (from the abdomen, flank, back) is typically performed prior to excision and reconstruction of the resultant Tajima 1 defects. Because the underlying, deeper structures such as the tendons and muscles are usually spared, functional outcomes are typically “good” or “normal” although follow-up, when available, remains limited in the literature (6 to 36 months) [679]. Functional preservation becomes increasingly challenging when Tajima 3 defects result following R0 resection for neoplastic processes, but can still be sufficiently addressed [10].
In terms of circumferential involvement in thermal burns resulting in Tajima 1 to 3 defects, the sequelae of chronic lymphedema [11] and scar contractures [12] oftentimes result in very poor functional outcomes due, in part, to involvement of the deep fascia, muscles and tendons, as well as the application of STSGs which have a high propensity for developing scar contractures. Similarly, electrical burns result in Tajima 1 to 3 defects with deep tissue involvement, especially that of the vasculature, which portends especially poor outcomes, both in the early and late postoperative period [1314]. This subgroup of patients has also seen the introduction and demonstrated utility of hand allotransplantation as a radical form of secondary treatment when conventional reconstructive surgery has failed to restore adequate function [12].
Mechanical trauma in the form of degloving, avulsion and/or crush injuries can oftentimes result in circumferential defects of the upper extremity due to machinery and even animal attacks. There was a noticeable trend in reconstructive techniques employed. Previously, the degloved/avulsed segment, if viable, would be applied back onto the affected extremity as a full thickness skin graft (FTSG) with or without additional STSGs. However, such an approach was invariably associated with the related complications of superficial flap necrosis (up to 42%) and scar contractures with limited functional restoration [1516]. Since then, various reconstructive techniques including fascial flaps [17], omental flaps [18], and fasciocutaneous flaps [19] have been reported with the potential to attain both excellent functional and cosmetic outcomes for Tajima 1 to 3 defects. This subgroup of patients has also seen the introduction of various adjuncts including tissue expansion and negative pressure wound therapy (NPWT) to improve the likelihood of achieving successful outcomes.
DISCUSSION
The current study has shown that circumferential defects of the upper extremity may develop subsequent to various etiologies, each of which will necessitate a different approach to management (Fig. 1).
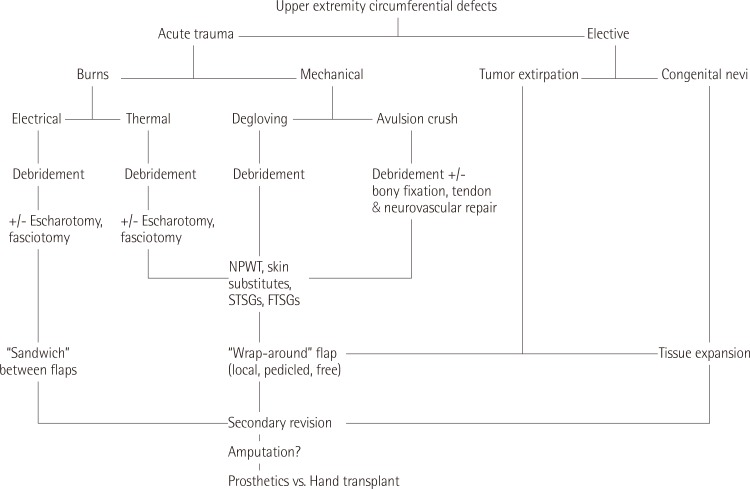
Algorithmic approach to upper extremity circumferential defects
Algorithmic approach to upper extremity circumferential defects. NPWT, negative pressure wound therapy; STSG, split thickness skin graft; FTSG, full thickness skin graft.
Circumferential sequelae resulting from large surface area burns, defects secondary to trauma or tumor resection, and conditions such as giant congenital pigmented nevi can now be better addressed in a staged setting through tissue expansion of regional and/or distant sites wherein the relative lack of healthy tissue obviates the option of local soft tissue coverage. Management of these complex defects in the acute setting is based on established principles and is detailed elsewhere [20].
One of the principal challenges of complete circumferential defects is the risk of proximal kinking of the pedicle during inset, leading to flap compromise and even, loss [21]. In the setting of electrical burns, the higher risk of associated thrombotic and aneurysmal complications [22] have led to the design of multiple pedicled flaps, rather than a single free tissue transfer, so as to “sandwich” the defect circumferentially and to create a tight seal to minimize the risks of deadspace and infections. However, with increasing knowledge of flaps and vascular anatomy, recent studies have shown that carefully designed fascial and fasciocutaneous flaps can be applied reliably in complete “wrap-around” fashion [1719].
While emergency free flaps used to be the maxim when underlying bone and tendons are exposed (i.e., Tajima 3 defects) [23], recent evidence suggests that NPWT in the form of vacuum assisted closure (VAC) devices may potentially help simplify the final reconstructive option (i.e., STSGs or transposition flaps rather than free tissue transfers) required without compromising on the final clinical outcome by promoting granulation tissue formation to prepare the wound bed for definitive closure [5]. Moreover, there are now increasing reports on the use of VAC devices in a circumferential manner in burns [242526] and lower extremity degloving [2728]. Additionally, VAC dressings have proven utility in helping to secure and improve STSG take [29], and has been used in clinical hand burns to prevent further progression of thermal injury through increased perfusion and reduction of edema [30]. Taken together, NPWT may, presumably, confer a similar benefit in traumatic, upper extremity circumferential defects requiring soft tissue reconstruction.
When donor sites for further STSGs are unavailable due to concomitant injuries or existing co-morbidities preclude lengthy surgeries, delayed wound closure can be achieved by using dermal regeneration templates such as Integra first due to its wide availability, followed by STSGs in a secondary setting as demonstrated by Lohana et al. [31] where upper limb involvement was most common (45%) in total body surface area burns of up to 64%. Similar to STSGs, bolstering of these skin substitutes with VAC therapy is well-described [29].
Finally, despite much effort and advancement in upper limb preservation following circumferential injury, the resultant scarring from STSGs or burn contractures may severely compromise and limit the final extent of functional recovery despite multiple secondary revision procedures such as scar revisions, contracture releases, tendon transfers, tenolysis etc. With the advent of vascularized composite allotransplantation (VCA), such residual circumferential defects and the resulting sequelae can now be comprehensively addressed through upper extremity transplantation [12]. Such functional outcomes in VCA are at least equivalent to, if not superior to the use of prosthetics whereby their usage is often abandoned altogether in up to a third of upper extremity amputees [32].
There remains a paucity of literature however, regarding postoperative management of circumferential upper extremity defects. Indiscriminate pressure from applying a circumferential dressing can result in arterial occlusion and/or venous congestion to the flap, concluding in partial or complete flap loss [33]. Mashhadi and Loh [34] described a useful method of applying a dressing, which involves interposing a regular 6-inch crepe bandage over the top of the wound covered with plain gauze. Following this, the authors apply the circumferential bandage. The 6-inch crepe bandage is removed before the final wrap, therein allowing an extra space between the flap wound and bandage hence creating enough slack. This ensures the dressing is secure enough to protect the flap while concurrently reducing the risk of occlusion. Margulis et al. [35] have established a postoperative protocol of a circumferential adhesive bandage over the arm, shoulders and trunk for several days, with gradual increments of motion ranging over 7 to 10 days. Ultimately, adherence to the basic tenets of postoperative wound care in reconstructive surgery will provide favorable outcomes.
Taken together, our article provides a review of the currently available evidence underlying the management of circumferential upper extremity wounds that may result from various etiologies. The algorithm for managing these complex cases involves an approach with judicious debridement of possibly contaminated and necrotic wounds; cognizance of the various reconstructive options with selection of a suitable modality based on the location, size, depth and etiology of the defect; and finally, postoperative management paying particular attention to dressing pressure and graduated motion increments of the affected limb to optimize functional outcomes which are paramount for the upper extremity. NPWT with skin grafting and tissue-expanded flaps have been employed to reasonably good effect but VCA may yet hold the greatest promise for patients with severe circumferential defects not amenable to conventional reconstructive methods should the associated immunological concerns be adequately addressed [36].
Notes
No potential conflict of interest relevant to this article was reported.