Prognosis of Full-Thickness Skin Defects in Premature Infants
Article information
Abstract
Background
In the extremities of premature infants, the skin and subcutaneous tissue are very pliable due to immaturity and have a greater degree of skin laxity and mobility. Thus, we can expect wounds to heal rapidly by wound contraction. This study investigates wound healing of full-thickness defects in premature infant extremities.
Methods
The study consisted of 13 premature infants who had a total of 14 cases of full-thickness skin defects of the extremities due to extravasation after total parenteral nutrition. The wound was managed with intensive moist dressings with antibiotic and anti-inflammatory agents. After wound closure, moisturization and mild compression were performed.
Results
Most of the full-thickness defects in the premature infants were closed by wound contraction without granulation tissue formation on the wound bed. The defects resulted in 3 pinpoint scars, 9 linear scars, and 2 round hypertrophic scars. The wounds with less granulation tissue were healed by contraction and resulted in linear scars parallel to the relaxed skin tension line. The wounds with more granulation tissue resulted in round scars. There was mild contracture without functional abnormality in 3 cases with a defect over two thirds of the longitudinal length of the dorsum of the hand or foot. The patients' parents were satisfied with the outcomes in 12 of 14 cases.
Conclusions
Full-thickness skin defects in premature infants typically heal by wound contraction with minimal granulation tissue and scar formation probably due to excellent skin mobility.
INTRODUCTION
Premature infants are live-born babies delivered before 37 complete weeks of gestation [1,2]. Their skin is still thin, reddish, and wrinkled with a small amount of subcutaneous fat due to immaturity [1]. Thus, the skin is softer and more pliable, resulting in a greater degree of skin laxity and mobility than a full-term infant has [2]. Full-thickness skin defects usually heal by such processes as granulation tissue formation, wound contraction, and marginal epithelialization [3-5]. Wound contraction reduces the size of the defect without the formation of new granulation tissues and accelerates the healing process better than epithelialization or scar formation does. The regional differences in wound contraction are probably due to relative differences in skin laxity [5]. We hypothesizedhat skin wounds in premature infants would close rapidly with less scar formation than in adults, and information about their wound healing could be applied to general wound management. The purpose of this paper is to investigate the prognosis of full-thickness defects in premature infants' extremities and their clinical significance.
METHODS
The current study consisted of 13 premature infants (14 cases) with full-thickness skin defects due to extravasation of total parenteral nutrition (Table 1). The gestational ages at birth ranged from 28 weeks and 4 days to 35 weeks and 6 days. The timing of extravasation was 14 to 50 days after birth.
Local and systemic treatments were performed after the infants were referred from the Department of Pediatrics. For local treatment, conservative management was performed and the defects healed by secondary intention. After cleansing the wound with normal saline, a sufficient amount of an antibiotic and anti-inflammatory ointment mixture was topically applied to the whole region of the wound site, followed by a saline-moistened gauze dressing. This treatment was performed twice a day during the acute phase and once a day during the convalescent phase. The necrotic tissue was removed when it was clearly demarcated. For systemic treatment, we administered prophylactic antibiotics during the acute stage and ascorbic acid mixed with fluids at a daily dose of 50 to 120 mg as the antioxidants. After wound closure, topical oil moisturization and mild compression were applied until the scars were stabilized.
We reviewed the size of the defect, duration of closure, healing pattern, and shape of the scar from the medical records and photographs. At a final follow-up visit, we evaluated the degree of the parents' satisfaction with the treatment outcomes by showing photographs of the initial defects and the final scar. The degree of satisfaction was graded at one of four levels: excellent, good, fair, and poor.
RESULTS
Among all of the 14 cases of full-thickness defects in the 13 premature infants, the defects were present on the hand or the wrist in 5 cases, on the foot or the ankle in 8 cases, and on the elbow in 1 case. The size of the defects ranged from 0.5 cm to three-fourths of the longitudinal length of the dorsum of the hand or foot. The defects had completely closed 14 to 55 days after injury. The follow-up periods ranged from 3 to 41 months (Table 1).
The vast majority of the defects closed by wound contraction. The direction of wound contraction was usually parallel to the longitudinal axis of the extremity, that is, vertical to the relaxed skin tension line (RSTL). The geometric shape of the defect gradually changed to elliptical. The shapes of the scars were pinpoint in 3 cases, linear in 9 cases, and round in 2 cases.
The defects smaller than 1 cm2 healed by wound contraction without granulation tissue formation and resulted in pinpoint scars (Fig. 1). In the linear scar cases, the defects were healed by wound contraction with no or minimal granulation tissue formation. In cases with no granulation tissue, the wound was covered using a whitish devitalized patch until wound closure. Minimal granulation tissue did not adhere to the marginal skin and slightly protruded with a shiny surface similar to one in a chronic wound of a pressure sore, and wound contraction readily occurred. Additionally, marginal epithelialization was extremely minimal. The linear scars were always parallel to the RSTL and rapidly transformed into mature scars (Fig. 2).
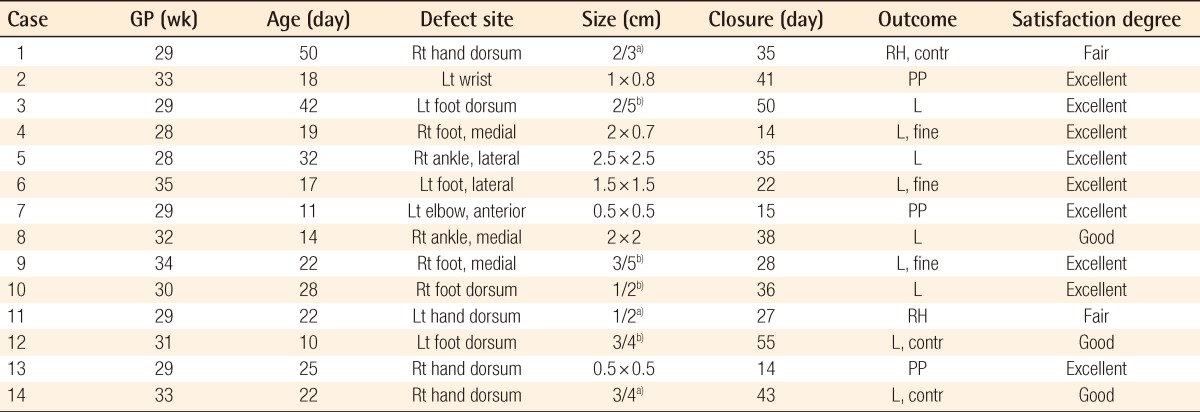
A case of a pinpoint scar
(A) Full-thickness skin defect 34 days after extravasation injury. (B) Eighteen months after treatment.
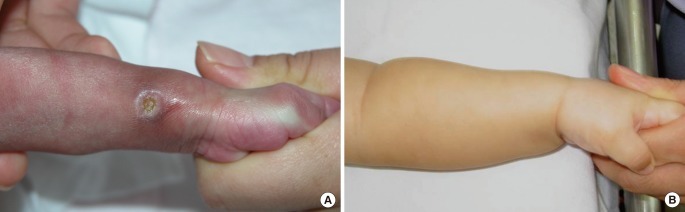
The cases of linear scars
(A) Extravasation on the left dorsum of the foot. A full-thickness skin defect was noted 18 days later. (B) Three months after treatment, there was a linear scar without contracture. (C) A full-thickness skin defect of three-fourths of the hand dorsum in longitudinal length. (D) Five months after treatment, a linear scar with mild contracture was observed, but no functional deformity remained.
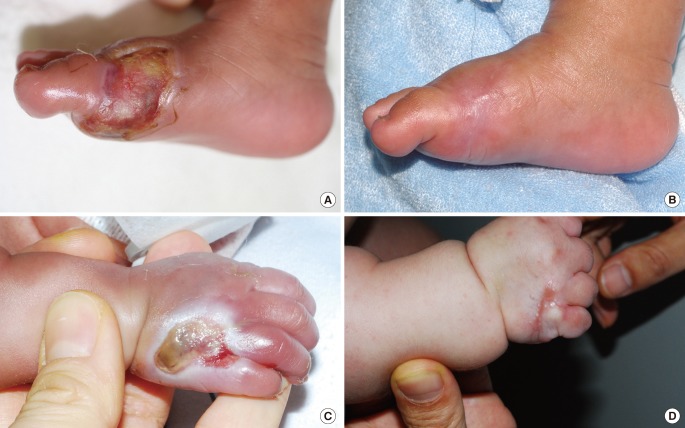
Two cases resulted in a round scar (Fig. 3). In these cases, initial wound care was delayed or intensive management was neglected. During wound contraction, beefy-red granulation tissue appeared on the wound bed and adhered to the marginal skin. Consequently, it was covered by marginal epithelialization. After wound closure, the round scars became gradually erythematous and slightly hypertrophic. The hypertrophic scars improved after intralesional steroid injection and compression, and eventually converted to slightly thick scars.
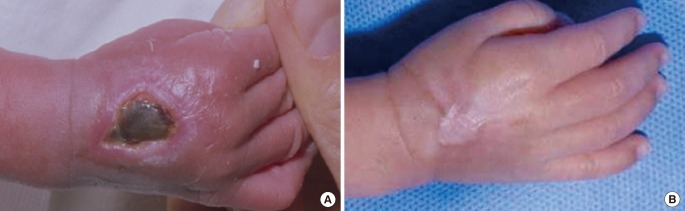
A case of a round hypertrophic scar with mild contracture
(A) A full-thickness defect of two-thirds of the right hand dorsum in longitudinal length 18 days after extravasation injury. (B) Ten weeks after treatment.
Mild contracture due to wound contraction was observed in 2 cases with linear scars on the dorsum of the hand and foot (Fig. 2C, D) and one case with a round scar on the dorsum of the hand (Fig. 3A, B). All of these occurred in defects that were over two-thirds of the longitudinal length of the dorsum of the hand or foot. Despite the contracture, there was no functional limitation of motion of the fingers or toes.
The degree of the parents' satisfaction was excellent in 9 cases of pinpoint scars and linear scars, good in 3 cases of depressed or mild contracted linear scars, fair in 2 cases of round hypertrophic scars, and poor in 0 cases.
DISCUSSION
Cutaneous closed wounds heal without scar formation in the early gestational fetus [5-8]. The fetal skin grafts heal wounds without scarring after transplantation to the subcutaneous tissue (internal environment) of adult mice [9]. When the adult skin is transplanted onto a fetus, the adult skin heals with scar formation and fetal skin heals without scarring [10]. Thus, it has been persistently argued that amniotic fluid (external environment) and fetal serum (internal environment) are not required for scarless healing [5,9], and scarless repair appears to be inherent to the fetal tissue and probably depends on factors associated with skin development [5].
Premature infants, delivered before 37 completed weeks of gestation, have premature skin (red, thin, smooth, shiny, and wrinkled with lanugo), less subcutaneous fat, and lower muscle tone than a full-term infant [1,2]. Thus, their skin is also very soft and pliable and has a high degree of skin laxity and mobility than a full-term infant. Additionally, the skin has a functionally immature epidermal barrier with significant transepidermal water loss [2,11,12]. The maturation of the skin barrier for functional adaptation to the extrauterine environment is fulfilled in 2 to 4 weeks, but dermal microcirculation continues to develop even beyond the neonatal period [13]. This may cause dry skin, vulnerability to trauma, and rapid onset of microbial colonization. Due to this skin immaturity, special skin care for moisturization (external environment) is required for wound healing and epidermal barrier repair [11].
In the current study, wound healing of the full-thickness skin defects due to extravasation injury in the premature infant extremities usually seemed to have several characteristics of less inflammatory response, less granulation tissue formation, maximum wound contraction, minimal epithelialization, and consequently minimal scar formation.
Wound contraction is a process in which the surrounding skin is pulled circumferentially toward an open wound [1-3]. This process is an important part of wound healing because there is a dramatic decrease in the defect size without new tissue formation. It accelerates wound closure more than epithelialization itself and makes the scar size smaller. The amount of contraction is related to the wound size and skin mobility [3]. Animals have a much greater capacity for wound contraction than humans do. Most mammals with a panniculus carnosus have a plane of low resistance between the musculoskeletal tissue and the skin, which allows maximum skin mobility and contraction. Therefore, they do not have any excessive scars [2]. In humans, wound contraction is the greatest in the trunk and external genitalia, but the least in the extremities [2,7]; this is probably due to skin mobility. Thus, wound contraction in adult human extremities (especially the hand and foot) can produce hypertrophic scars and/or scar contracture.
In premature infants, however, the skin is still thin, reddish, and wrinkled with a small amount of subcutaneous fat due to immaturity [8]. Thus, the skin has less tensile strength and greater degree of laxity and mobility. In this study, closure of the defects was mainly dependent on wound contraction due to their histophysiological properties even in the extensive full-thickness defects of the dorsum of the hand and foot where the degree of contraction is relatively low in the adult.
Inflammation and granulation tissue formation play key roles in adult wound healing, but not in that of an early fetus [3-10] because the amount of inflammation strongly correlates with the amount of scar formation [6]. Most of the inflammation-inducing or -accelerating factors arise from the external environment (pH, bacteria, chemicals, dryness, and foreign bodies, etc.) or internal environment (blood cells and plasma). The fetal skin grafts heal wounds with scar formation after transplantation onto the skin of adult mice exposed to the air (external environment) [8]. After 24 weeks of gestation, human fetal amniotic fluid (external environment) does not reflect fetal plasma (internal environment) because of urine accumulation and reduced diffusion of the fetal plasma [1]. In late gestation, it is after a transition period, after 24 weeks when follicular keratinization is completed [14], that cutaneous wounds heal with scar formation [5,7]. Although the fetal environment alone cannot induce scarless healing in adult skin [10], these results suggest that scarless repair appears to depend on the external environment to some extent. Thus, our concept of local wound management is to create an optimal external environment for the wound.
In the current study, most of the full-thickness skin defects in the premature infants, who received both initial and continuous wound management, had no or minimal inflammation or granulation tissue formation, which resulted in pinpoint or linear scars. The minimally formed granulation tissue did not show a beefy-red appearance but was slightly protruded with a shiny surface despite of no bacterial growth in the wound culture. It was also not adhered to the marginal skin until wound closure. In contrast, there was an inflammatory response around the wound in the case of delayed initial treatment or discontinued wound management. Consequently, granulation tissue increased, gradually turned to a beefy-red appearance, and adhered to the wound margin during wound contraction. After epithelialization, this led to the appearance of a hypertrophic scar. Thus, we guessed that more granulation tissue, especially in a beefy-red appearance, probably inhibits wound contraction due to marginal adhesion and may result in an unsatisfactory round scar formation. In other words, reduction of the inflammatory response might play an important role in inhibiting granulation tissue and scar formation similar to fetal wound healing.
Local wound management in premature infants optimizes healing by maintaining a physiologic local wound environment, which is characterized by adequate moisture, normal body temperature, bacterial balance, and neutral to mildly acidic pH [12]. The moisture must be kept in balance to prevent both desiccation and maceration of the wound and surrounding skin [12]. We performed extensive local treatment of the whole area including the adjacent region and defect site using oil- and saline-moistened dressings. This was to protect and soften the wound and the surrounding skin. The 12 cases, which underwent proper treatment, were closed mainly by wound contraction, resulting in a satisfactory appearance of the pinpoint or linear scars without any significant functional deformity. In addition, the direction of wound contraction was usually vertical to the RSTL. Consequently, the linear scars were situated parallel to the RSTL. Furthermore, contracture was mild even in the extensive defects, which extended over two-thirds of the longitudinal length of the dorsum of the hand or foot. This suggests that enhancement of regional skin mobility is important for minimizing external scarring and for promoting wound contraction and closure. Thus, local treatment may need to be performed extensively up to the adjacent regions for the defect to heal with a satisfactory outcome until the scar is stabilized.
The total number of scars was inversely related to the gestational age and directly related to the duration of intensive care due to the significant lesions caused by the multiple invasive procedures for their survival [15]. To reduce the frequency and severity of skin damage, a more careful process of wound closure is needed. We expect that intensive wound management for moisturization and reduction of the inflammatory response could help enable the successful treatment of other defects of premature infants and extravasation wounds. This concept may also be applied to general wound management in adults.
This study had limitations, including the lack of an animal model research basisand a quantitative biochemical study. The quantitative extent of wound contraction in premature infants compared with those of adults remains somewhat unclear. Therefore, more studies are needed to reveal more precisely the wound healing process of premature infants.
Notes
This article was presented at the 1st Research and Reconstructive Forum on May 12-13, 2011 in Deajeon, Korea.
No potential conflict of interest relevant to this article was reported.