Using a Thermal Imaging Camera to Locate Perforators on the Lower Limb
Article information
Abstract
Reconstruction of the lower limb presents a complex problem after skin cancer surgery, as proximity of skin and bone present vascular and technical challenges. Studies on vascular anatomy have confirmed that the vascular plane on the lower limb lies deep to the deep fascia. Yet, many flaps are routinely raised superficial to this plane and therefore flap failure rates in the lower limb are high. Fascio-cutaneous flaps based on perforators offer a better cosmetic alternative to skin grafts. In this paper, we detail use of a thermal imaging camera to identify perforator ‘compartments’ that can help in designing such flaps.
INTRODUCTION
Reconstruction of the lower leg after skin cancer excisions can be challenging due to actinic damaged thinned-out skin that is friable, and poor vascularity in patients with diabetes or other diseases that impact the vascular system. Even in healthy individuals, the lower limb presents a specific challenge due to the ‘surgical plane’ of the lower limb. When it comes to closing large defects on the lower limb, random pattern cutaneous flaps are also limited by the arc of rotation and by the resulting decrease in bacterial resistance, as well as a general rule of a 2:1 ratio between the length and base of the flap [1].
Skin flaps, especially random cutaneous flaps have fared poorly on the lower limb and probably because, as literature reviews have shown, most flaps have been raised superficial to the deep fascia [2]. Haertsch [2] conducted a dye-injection study on 22 post-mortem legs and demonstrated the role of the fascial plexus. His study showed that the fascial plexus communicated with the sub-dermal plexus and sometimes with a vascular plexus on the undersurface of the deep fascia. His study confirmed, that for practical purposes, the ‘surgical plane’ of the leg lies deep to the deep fascia.
Given this ‘surgical plane’ of the lower limb, many surgeons have designed perforator-based flaps and have used Doppler probes to try and incorporate perforators into the base of a flap [3]. While the hand-held Doppler device has often been used to try and identify perforators in the lower limb, review of the literature suggests that that most of such unidirectional Doppler flowmetry analyses for perforator-based flaps have been done predominantly on the trunk [3]. Authors have noted that while a handheld Doppler can demonstrate the direction of blood flow, it fails to determine the size of the vessel or its flow volume—thereby resulting in high false-positive or false-negative results at extremities, especially the lower limb [3]. Using a color Doppler can obviate some of these risks, but all Doppler devices are operator dependent—both in terms of skill, and prior anatomical knowledge of skin perforator locations [3].
Given the variability of lower limb perforators and the difficulty in identifying specifics islands of tissue that can be perfused by reliably-identifiable perforators, recent studies have attempted to use computed tomography (CT) scans to study locations of perforators—with the authors noting that CT angiography was able to show perforators with high resolution and accurately demonstrate vessel size, location and course [4]. It is now established that in most cases, distal arterial perforators have veins accompanying them–in general, a vein lies inferior to the artery; when there are two veins, they often tend to interconnect around the artery.
IDEA
While island flaps such as the V-Y advancement island flap, the Bezier flap and the Keystone Design Island flap are often mentioned in the context of perforator-based island flaps of the lower limb, one issue with conventional island flaps is that when tissue is advanced or transposed, a twist of the tissue occurs and this increases the external pressure on the venous supply. It is a well-known plastic surgical dictum that fascio-cutaneous flaps, once ‘islanded,’ are solely reliant on the venae comitantes for venous drainage. Therefore, it is important to know where reliable venous perforator drainage exists as this can help plan the location of such islands. We therefore set out to study lower limbs using a thermal imaging camera to see we could find reliable perforator locations in the lower limbs that could help design perforator-based fascio-cutaneous flaps.
Innovation
For this study we used a thermal imaging camera (FLIROne for iPhone, manufactured by Flir Systems Inc., Wilsonville, OR, USA) to take images of lower limbs prior to cutaneous surgery. This device contains a non-contact spot temperature measurement with automatic shuttering, and has been designed to capture a thermal reading of photographic images—and therefore in the past, has been primarily used in detecting leaks in buildings.
The technique for using the FLIROne Camera to venous perforators has already been defined. Suphachokauychai et al. [4] have already provided guidelines for the use of these cameras to obtain accurate results. They noted that firstly the camera should be used on a dry surface. Ideally the measuring distance should be 100 cm and the thermal range should be locked at 25℃–30℃. These authors note that this technique has become their favored one for identifying vascular perforators, allowing them the freedom to design a free-style perforator flap [4]. We decided to follow these parameters for our measurements.
The protocol for this study and informed consent were approved by an institutional review board, in our case the health and disability ethics committees (HDECS) Ethics Approval Committee, and all subjects gave informed consent: all ethics approvals for any studies have been approved by the relevant authorities: New Zealand Health and Disability Ethics Committees (HDEC Reference number 15/CEN/113); Australia: Queensland Institutional Human Ethics (Approval No. 2015001550).
We know from the manufacturers of FLIR one that the palette of colors exhibits the following pattern: the coldest areas are displayed in blue, the hottest areas in white, with red and yellow in between [5]. We also know from previous studies that the venous temperature is usually higher than the temperature in the artery [6]. And when it comes to tumors and heat conduction through the surrounding tissue, the tumor heat reaches the skin surface or it is transported indirectly with the venous blood flow to the skin [6]. Therefore venous perforators are hotter than arteries in the legs, we would expect the veins to display a whitish palette and this was what we noted.
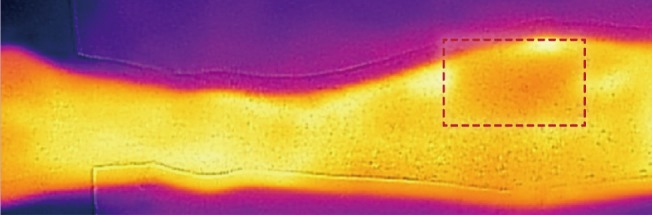
A total of 23 cases formed part of this study—most of the lesions, 16 were pretibial lesions, 4 were located closer to the ankle and 3 were located on the calf. We found the use of thermal imaging camera technically easy and with insignificant user-variation. It was easy to identify thermal patterns for venous perfusion (whiteness) and arterial suffusion (redness) as noted in a case where a fasciotomy had been performed (Fig. 1). What was immediately apparent was the remarkable consistency of venous perforator ‘islands’ on the lower limb—we noted them in three particular locations that corresponded with previously noted locations of perforator clusters in the lower limb.
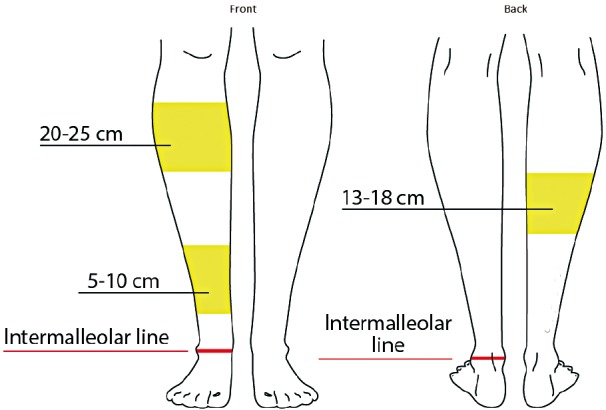
We marked out the regions proximally from the inter-malleolar line and venous perforators were shown to be concentrated in certain segments (Fig. 2). The first cluster was at 5–10 cm from the inter-malleolar line that corresponded with known locations of anterior tibial artery perforators that originate between the tibia and tibialis anterior muscle. Anteriorly, the main larger cluster was at 20–25 cm proximal to this inter-malleolar line, and this corresponds with perforators that originate via the anterior peroneal septum, between the extensor digitorum longus and the peroneus longus muscles (Fig. 2). Posteriorly on the calf, there was a cluster at 13–18 cm—corresponding with known perforators from the peroneal artery that course through the posterior peroneal septum and these are indicated in the image (Fig. 3).
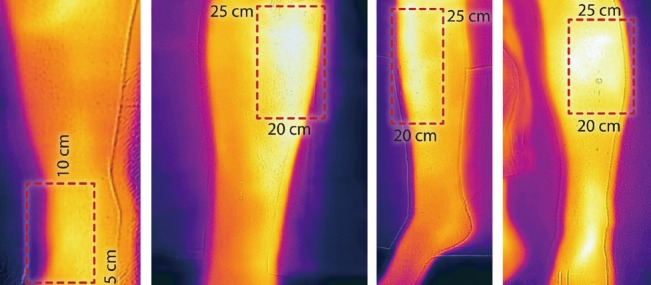
Anterior tibial artery perforators
Perforators from anterior tibial artery are located in 2 distinct segments, most prominent 20–25 cm from inter-malleolar line (whiteness indicates venous perfusion).
Peroneal artery perforators
Posteriorly calf perforators from the peroneal artery are located at a cluster 13–18 cm from the inter-malleolar line.
We must note that in using this technique, like the use of handheld Dopplers or ultrasound, there is a degree of operator experience required, knowing the optimum imaging distance; however, parameters are easier to define here in this case, and the results were both consistent and reproducible. We set out of mark out ‘perforator islands’ as a guide for cutaneous surgeons. We essentially have mapped locations of blocks of tissue with adequate venous perfusion that could be utilized for island flaps.
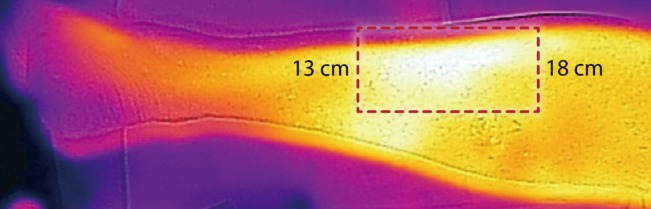
We did not set out to identify specific perforators, which we believe is not achievable consistently. However, as noted in the image (Fig. 4) operators have successfully used this to raise island flaps such as the Bezier Island Flap incorporating venous perforators. In the image (Fig. 4) the red dot is the tumor and the white islands are the perforators of the flap from the donor side of the Bezier flap.
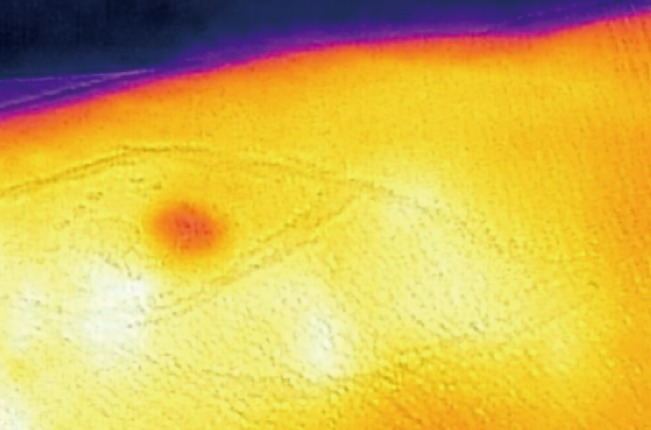
Thermal imaging to plan perforator flap
Using thermal imaging to plan a Bezier Island Flap (image courtesy Dr. Tony Azzi, Newcastle, Australia).
In studying vascular disorders, many authors have noted the equivalence between thermography and hand-held Dopplers [7].
DISCUSSION
As we have noted earlier, venous drainage plays a major part in ensuring island flap viability in the lower limb, and therefore there is a need to identify locations of suitable blood supply—from whence an island flap can be mobilized from. Detailed cadaveric dissections have been done by other authors [5], who also noted that major perforators of the lower leg were located in three distinct clusters.
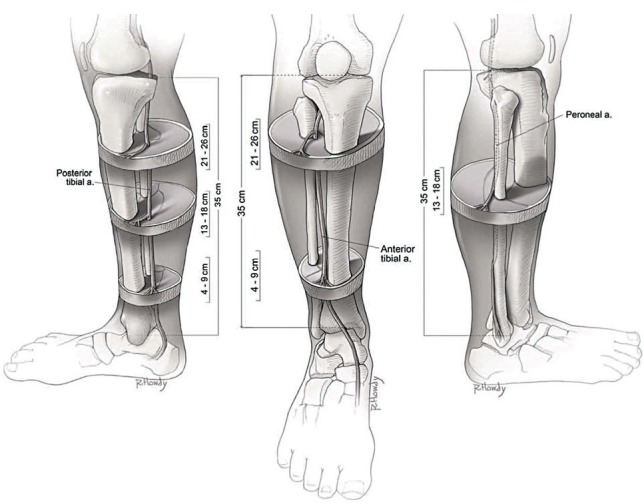
In summary, the lower limb has three main clusters of perforating veins (Fig. 5) that are especially useful in designing island fascio-cutaneous flaps, where venous drainage can be the defining criterion for viability of the flap. We undertook a literature review of cadaveric dissections that had previously identified known perforator locations after our study was completed, and were pleasantly surprised to note that our findings correlated well with the results noted by Schaverien and Saint-Cyr [8] in cadaveric dissections. And also, these correspond with locations that island perforator flaps are generally more successful. In the illustration (Fig. 6) [8] the authors noted that perforators are found between the flexor digitorum longus and soleus at three intervals, with the largest perforators found in the middle cluster (center image). Illustration of the lower leg shows the location of reliable perforators from the anterior tibial artery, the proximal cluster, from 21 to 25 cm, that is found within the anterior peroneal septum between the extensor digitorum longus and the peroneus longus. The distal cluster, from 4 to 9 cm, was found between the tibia and the tendon of the tibialis anterior muscle (right image). The illustration of the lower leg also reveals the location of reliable perforators from the peroneal artery. The cluster is found between 13 and 18 cm, within the posterior peroneal septum. Proximally, the perforators emerged through the soleus or peroneus longus muscles, and distally they were found between the flexor hallucis longus and the peroneus brevis. As illustrated in Figs. 2 and 3, our study using thermal imaging achieved a remarkable correlation with these cadaver dissection studies demonstrating the usefulness and validity of our technique.
Venous ‘islands’ of the lower limb
The lower limb has three main clusters of perforating veins that are especially useful in designing island fascio-cutaneous flaps.
We are presenting our innovative method of using thermal imaging to identify vascular supply—as it is the first time this has been used in this manner—and this can greatly help in the planning of island flaps as this offers a general guide for those undertaking skin cancer surgery (the specifics of such flaps we have used in these situations will be the subject of another paper). Further, while previous researchers have had to undertake cadaveric dissections, Doppler imaging or arrange CT scans [9] to locate perforators, our technique was simple, was performed easily prior to and during surgery, and was reliably reproducible by different operators, even if there is some user skill required. In contrast Doppler techniques have been noted to have a 45% chance of producing a false positive overall, with an error-rate being higher amongst inexperienced surgeons [10]. The thermal imaging camera is therefore more cost effective and being a visual method, more efficient. The other advantage with our method is that, given some individual anatomical variations (clusters being more medial or lateral), we were able to locate perforator clusters prior to surgery and therefore individualize surgical flaps to achieve optimum results. Milton [11] noted in his experimental studies on island flaps that when a flap is ‘islanded’ it is the venous supply that matters and he famously wrote about lower limb island flaps saying, “An island is safer than a peninsula.” Therefore the ability to map the general locations of perforator islands is helpful in lower limb island flap surgery, and that was the intention behind this paper.
Notes
This paper resulted from a study that forms part of my Ph. D research project at the University of Queensland's School of Medicine and I would like to acknowledge my supervisors, Assoc. Professor Cliff Rosendahl and Professor John Windsor. And finally, I would like to thank Ryan Butler, Auckland University of Technology, for his help with photography to document this experiment.
This article was presented at the 8th annual HealthCert/University of Queensland Skin Cancer Conference and Masterclasses, July 28, 2016, Brisbane, Australia.
No potential conflict of interest relevant to this article was reported.