Thickness of Rectus Abdominis Muscle and Abdominal Subcutaneous Fat Tissue in Adult Women: Correlation with Age, Pregnancy, Laparotomy, and Body Mass Index
Article information
Abstract
Background
Rectus abdominis muscle and abdominal subcutaneous fat tissue are useful for reconstruction of the chest wall, and abdominal, vaginal, and perianal defects. Thus, preoperative evaluation of rectus abdominis muscle and abdominal subcutaneous fat tissue is important. This is a retrospective study that measured the thickness of rectus abdominis muscle and abdominal subcutaneous fat tissue using computed tomography (CT) and analyzed the correlation with the patients' age, gestational history, history of laparotomy, and body mass index (BMI).
Methods
A total of 545 adult women were studied. Rectus abdominis muscle and abdominal subcutaneous fat thicknesses were measured with abdominopelvic CT. The results were analyzed to determine if the thickness of the rectus abdominis muscle or subcutaneous fat tissue was significantly correlated with age, number of pregnancies, history of laparotomy, and BMI.
Results
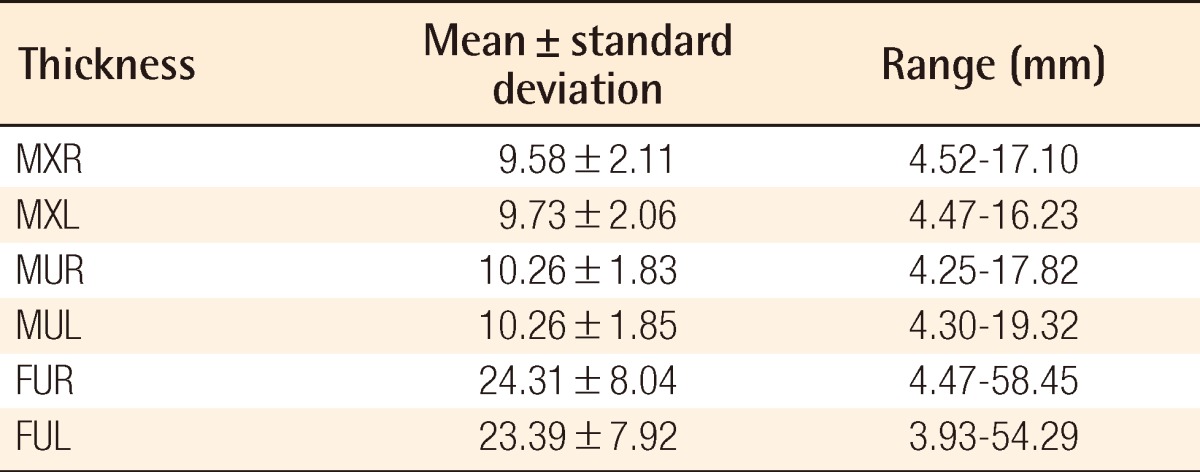
Rectus abdominis muscle thicknesses were 9.58 mm (right) and 9.73 mm (left) at the xiphoid level and 10.26 mm (right) and 10.26 mm (left) at the umbilicus level. Subcutaneous fat thicknesses were 24.31 mm (right) and 23.39 mm (left). Rectus abdominismuscle thickness decreased with age and pregnancy. History of laparotomy had a significant negative correlation with rectus abdominis muscle thickness at the xiphoid level. Abdominal subcutaneous fat thickness had no correlation with age, number of pregnancies, or history of laparotomy.
Conclusions
Age, gestational history, and history of laparotomy influenced rectus abdominis muscle thickness but did not influence abdominal subcutaneous fat thickness. These results are clinically valuable for planning a rectus abdominis muscle flap and safe elevation of muscle flap.
INTRODUCTION
Many developments have occurred in the use of the rectus abdominis muscle as a local or distant flap ever since Mathes and Bostwick [1] and Hartrampf et al. [2] identified the pedicled rectus abdominis muscle flap. Although the use of the pedicled rectus abdominis muscle flap has recently decreased due to the development of microvascular surgery, it is widely used when a free flap is unsuitable due to the poor condition of recipient vessels caused by radiotherapy or trauma, or due to the surgeon's choice [3-5].
While microvascular surgery with a perforator flap is a current trend, a few articles have been found that mention the importance of the thickness of the rectus abdominis muscle. When the rectus abdominis muscle flap is necessary, however, it is a clinically important factor for the safe elevation of the muscle flap and the safe coverage of the defect of the recipient site.
The thickness of the abdominal subcutaneous fat tissue is one of the important factors for fasciocutaneous or myocutaneous flaps using the rectus abdominis muscle. This is because much of the volumetric effect on the recipient site is from the subcutaneous fat tissue of the transferred flap such as the transverse rectusabdominis myocutaneous flap (TRAM) or the vertical rectusabdominis myocutaneous flap (VRAM).
The thicknesses of the rectus abdominis muscle and the abdominal subcutaneous fat tissue were measured using abdomenpelvis computed tomography (APCT).The patients' medical records such as age, gestational history, history of laparotomy, and body mass index (BMI) were reviewed.
METHODS
Subjects and factors
A total of 545 female patients (age range, 20 to 60 years) who visited the Department of Obstetrics and Gynecology and the Department of Family Medicine of the authors' hospital to have APCT scans taken between October 2010 and March 2012 were studied. We reviewed the patients' medical records such as age, height, weight, gestational history, and history of a laparotomy. When APCT had been performed on the patients several times, we used the results of the first scan in which all of the relevant information was recorded. The patients who had undergone radiotherapy were excluded.
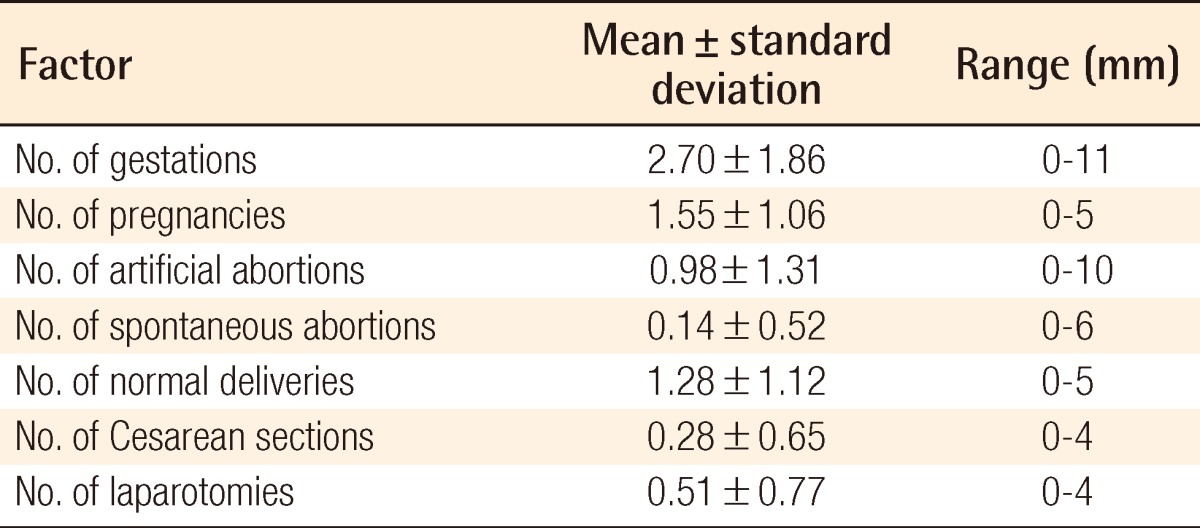
To investigate the influence of gestational history, the six clinical indices of number of gestations (G), number of live-born children (P), number of artificial abortions (AA), number of spontaneous abortions (SA), number of normal deliveries (ND), and number of Cesarean section deliveries (CS) until the applied age were obtained from their medical records.
History of previous abdominal surgery was investigated from their medical records. Operations such as appendectomy, laparoscopy, and pelviscopy, which had created a short scar less than 5 cm, were disregarded. Cases of abdominal surgery that left a scar longer than 5 cm were regarded in the study as cases of laparotomy. The type of laparotomy that the patients had experienced until the applied age, such as CS laparotomy, abdominal hysterectomy, exploratory laparotomy, cholecystectomy, orcolon cancer surgery, was noted, and the number of laparotomies (L) undergone was obtained.
Thicknesses of the rectus abdominis muscle and thickness of the subcutaneous fat tissue
The computed tomography (CT) images chosen for the study were analyzed. The thicknesses of the rectus abdominis muscle and abdominal subcutaneous fat tissue were measured with the picture archiving and communications system (PACS) viewer program (PiViewSTAR, Infinitt Healthcare Co., Seoul, Korea) and viewed on computer monitor: the caliper tool in the program was used after standardizing the grey scale to the abdomen modeunder ×100 magnification at 0.01 mm. The thickest part of the muscle at the two levels of the xiphoid process and the umbilicus was measured bilaterally. The thickness of the subcutaneous fat tissue was measured bilaterally at the point 5 cm lateral to the umbilicus. The measured thicknesses were defined as thickness of the right muscle at the xiphoid level (MXR), thickness of the left muscle at the xiphoid level (MXL), thickness of the right muscle at the umbilicus level (MUR), thickness of the left muscle at the umbilicus level (MUL), thickness of the subcutaneous fat tissue on the right side at the umbilicus level (FUR), and thickness of the subcutaneous fat tissue on the left side at the umbilicus level (FUL) (Fig. 1).
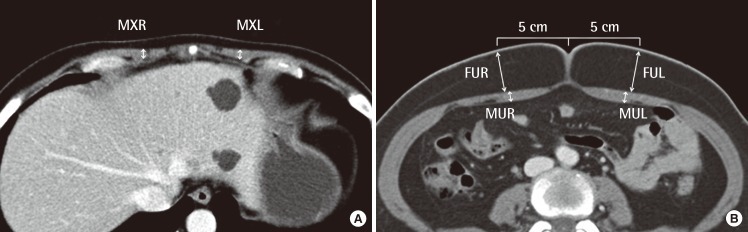
Two levels of measurement
The thickness of the rectus abdominis muscle and abdominal fat were measured with the picture archiving and communications system viewer program (PiviewStar) using the caliper tool from the program after standardizing the grey scale to the abdomen mode (×100). (A) Xiphoid level. (B) Umbilicus level. MXR, thickness of the thickest part of the right rectus abdominis muscle at the xiphoid level; MXL, t hickness of the thickest part of the left rectus abdominis muscle at the xiphoid level; MUR, thickness of the thickest part of the right rectus abdominis muscle at the umbilicus level; MUL, thickness of the thickest part of the left rectus abdominis muscle at the umbilicus level; FUR, thickness of the subcutaneous fat tissue on the right side at the umbilicus level at the point 5 cm to the right of the umbilicus; FUL, thickness of the subcutaneous fat tissue on the left side at the umbilicus level at the point 5 cm to the left of the umbilicus.
Statistical analysis
Statistical analysis was performed using Pearson's correlation coefficient to determine how factors such as age, BMI, G, P, AA, SA, and L were correlated with the thicknesses of the rectus abdominis muscle and the abdominal subcutaneous fat tissue. All statistical analyses were performed with statistical software (SPSS ver. 12.0, SPSS Inc., Chicago, IL, USA), and a value of P<0.05 was considered statistically significant.
RESULTS
Subjects
The average age was 43 years. The average BMI was 23.35 kg/m2 (range, 15.35 to 38.05 kg/m2). The data of the gestational history are listed in Table 1.
Thicknesses of the rectus abdominis muscle and the abdominal subcutaneous fat tissue
The thicknesses of the rectus abdominis muscle were measured as follows: MXR, 9.58±2.11 mm; MXL, 9.73±2.06 mm; MUR, 10.26±1.83 mm; and MUL, 10.26±1.85 mm. The thickness of the subcutaneous fat tissue at the umbilicus level was measured as follows: FUR, 24.31±8.04 mm; and FUL, 23.39±7.92 mm (Table 2). There were significant differences between MXR and MXL on the paired sample t-test (P=0.005): FUR and FUL (P=0.000). Only between MUR and MUL was there was no significant difference (P=0.110).
The correlations with various factors
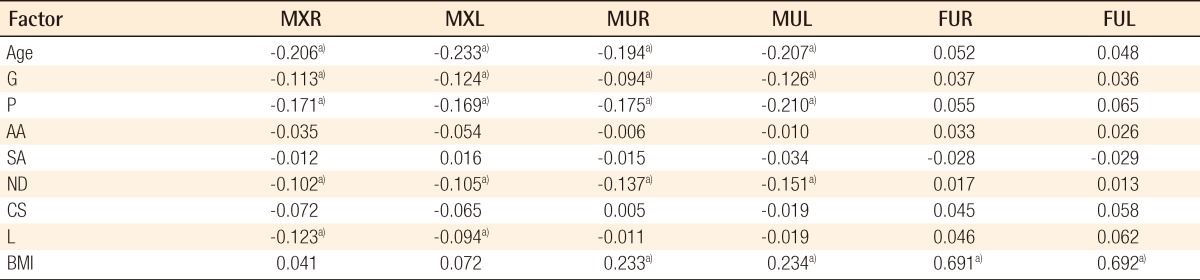
The Pearson's correlation coefficient test showed the following results. The age, G, P, and ND had significant negative correlations with MXR, MXL, MUR, and MUL. L had significant negative correlations with MXR and MXL. BMI had significant positive correlations with MUR, MUL, FUR, and FUL. All of the other combinations had no correlations (Table 3).
There were negative correlations between age and muscle thickness regardless of the side or the level (Fig. 2). In brief, rectus abdominis muscle became thinner with aging. However, the thickness of the abdominal subcutaneous fat tissue was not influenced by the age.
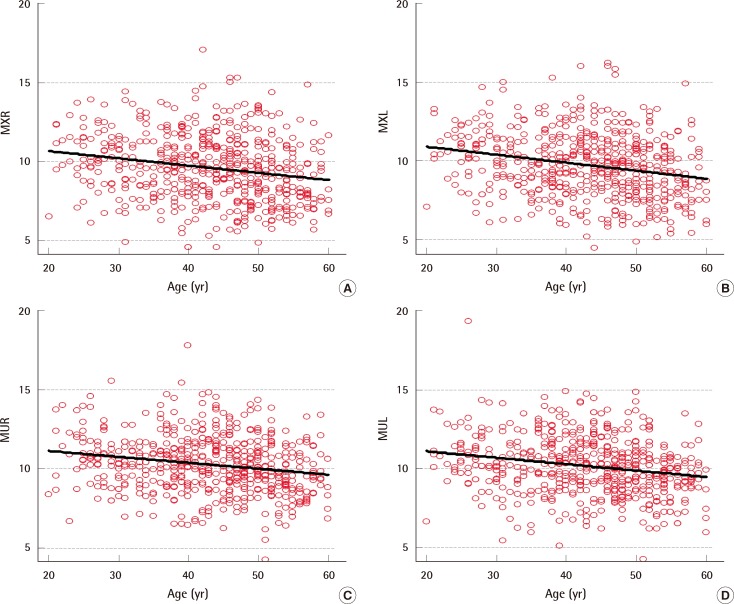
Scattered diagrams and the correlations of age
(A) Correlation between age and right rectus abdominis muscle thickness at the xiphoid level. (B) Correlation between age and left rectus abdominis muscle thickness at the xiphoid level. (C) Correlation between age and right rectus abdominis muscle thickness at the umbilical level. (D) Correlation between age and left rectus abdominis muscle thickness at the umbilical level. MXR, thickness of the thickest part of the right rectus abdominis muscle at the xiphoid level; MXL, thickness of the thickest part of the left rectus abdominis muscle at the xiphoid level; MUR, thickness of the thickest part of the right rectus abdominis muscle at the umbilicus level; MUL, thickness of the thickest part of the left rectus abdominis muscle at the umbilicus level.
Among the gestational factors, gravida, para, and normal delivery factors had a negative correlation with muscle thickness regardless of the side or level. This means pregnancy and normal delivery made the rectus abdominis muscle thin. However, abortion and CS delivery did not influence muscle thickness. None of the factors related with pregnancy influenced subcutaneous fat thickness.
Abdominal surgery negatively influenced the thickness of the upper portion of the rectus abdominis. The thicknesses of the lower portion of the rectus abdominis muscle and abdominal fat were not influenced. The thickness of the rectus abdominis and fat at the umbilical level increased when a woman became obese.
DISCUSSION
In rectus abdominis muscle flap for chest wall or breast reconstruction, there are three important roles of the thickness of the rectus abdominis muscle. First, a proper thickness of the rectus abdominis musclehelps ensure the flap elevation is safe. The deep superior epigastric artery (DSEA) and the deep inferior epigastric artery (DIEA), both of which are major pedicles of the rectus abdominis muscle flap, branch out to the perforators that pass beneath or inside or through the rectus abdominis musclefrom the ventral layer of the rectus sheath to the skin [6]. When the muscle is too thin, there is a possibility of injury of the pedicle, if the pedicle is exposed to tension such as from retracting hand-power during dissection. When the muscle is sufficiently thick, it prevents injury of the pedicle or the segment of the perforator because it bears more tension. Second, a proper muscle thickness can indicate the condition of the pedicle. If the rectus abdominis muscle is thick and healthy, it can be accepted, in that the muscle has sufficient blood supply and the pedicle is in good condition. Third, proper thickness of the muscle has a volumetric effect on the recipient site, which serves the original purpose of the coverage of the defect. In the case of chest wall reconstruction, for example, the muscle itself covers the defect. Thus, information on the thickness of the rectus abdominis muscle is clinically very important and can be helpful to a surgeon [7].
Rankin et al. [8] reported in their study of 86 women using ultrasonography that the thickness of the right rectus abdominis muscle was 10.2±1.6 mm. Kanehisa et al. [9] reported in their study using ultrasonography on 92 women that the muscle thickness decreased as the age increased due to the influences of age and sex on the abdominal muscle and the subcutaneous fat thickness. The muscle thicknesses in those two studies could be measured to the close at 0.1 mm at best. No other factor of influence besides age and sex was considered. The negative correlation between age and the subcutaneous fat thickness in their study was not shown in this study. However, in this study, the muscle thickness could be measured at 0.01 mm under ×100 magnification of CT image in the PACS viewer program. The age, gestational history, history of laparotomy, and BMI were considered to be factors that could influence the muscle and fat thickness. The sample sizes of those two studies were much smaller than that of this study. Thus, this study can be regarded as having used a more detailed method of measurement and a better sample, and having more reliable results.
Suh et al. [7] studied the thickness of the muscle, the subcutaneous fat tissue, and the skin at various sites, which could be donor sites in a flap surgery for reconstruction, and emphasized the importance of the thicknesses of the subcutaneous tissue from the surgeon's point of view.Their study showed the thickness of the subcutaneous fat at the rectus abdominis was 10.2 mm: the same as our results.
Age, gestational history, history of laparotomy, and BMI were applied as factors in this study is for the following reasons. Age can affect many clinical factors. As a patient's age increases, she is more likely to have experienced pregnancy or delivery and surgery. During pregnancy, the rectus abdominis muscle and the subcutaneous fat tissue has undergone pressure from the inside by the increased abdominal volume. The abdominal muscle and subcutaneous fat tissue become thinner until delivery. A mechanical force affects the thicknesses of the rectus abdominis muscle and the subcutaneous tissue during pregnancy. Laparotomy can cause an injury to the blood supply, in turn causing hypotrophy of the rectus abdominis muscle. Scar formation after laparotomy can cause soft tissue adhesion that can change the thicknesses of the abdominal muscles and subcutaneous fat tissue.
The thicknesses of the rectus abdominis muscles on both sides at the xiphoid level showed a negative correlation with the number of laparotomies. That means, with more laparotomies the upper portion of the rectus abdominis muscle becomes thinner. This is possibly because the direct injury to the rectus abdominis muscle or the injury to DSEA, DIEA, or their perforators after laparotomy can decrease the blood supply. The decreased blood supply can cause hypotrophy of the rectus abdominis muscle and the subcutaneous fat tissue. Thus, laparotomy can have a negative hemodynamic effect on the rectus muscle and the subcutaneous fat tissue and cause them to decrease in thickness.
Among a total of 545 patients, 6 rectus abdominis muscles (4 right sides and 2 left sides) at the xiphoid level were very thin at less than 5 mm (Fig. 2). When the pedicled TRAM flap is considered for reconstruction, an extremely thin muscle pedicle can cause circulation problems. If plastic surgeons do not check the thickness of the muscle before pedicled TRAM flap, it could interfere with the success of their operation in some cases.
The resulting positive correlation between the thicknesses of the rectus abdominis muscle and the subcutaneous fat tissue could be thought to be a concern in the volumetric effect of the recipient site in the pedicled TRAM flap or the free TRAM flap. This is because the subcutaneous fat tissue of the flap plays a part in contouring the soft tissue in reconstruction [5,7]. The extensive abdominal subcutaneous fat tissue of an obese patient is not a desirable condition because obesity increases the risk of complications. However, clinically, except in cases where a patient is too obese or the abdominal subcutaneous fat tissue is too thick for undergoing surgery, the resulting positive correlation between the thicknesses of the rectus muscle and the subcutaneous fat tissue is clinically meaningful. Thus, BMI, which is an index of obesity, can be an index for predicting the thickness of the abdominal subcutaneous fat tissue or the postoperative survival of the TRAM flap or the VRAM flap. For example, an obese patient with a BMI greater than 25.8 kg/m2 is more likely to experience a postoperative complication after a TRAM flap [10].
In this study, some numerical data on the thicknesses of the rectus abdominis muscle and the abdominal subcutaneous fat tissue were derived, and the statistical analysis of the data showed that the thickness of the rectus abdominis muscle had negative correlations with the age, the number of deliveries, and the number of laparotomies. At the xiphoid level, the thickness of the rectus abdominis muscle is important because DSEA, which is the pedicle commonly in the pedicled TRAM, is located beneath the rectus abdominis muscle. Previous laparotomy can cause an injury to the pedicle, and decreased blood flow can cause the upper portion of the rectus abdominis muscle to thin.
Complicated pregnancies such as a hydramnios, overweight newborn baby, and twins or triplets can influence the thickness of the rectus abdominis muscle significantly. This is the reason prospective studies on these factors are needed.
The significance of this study can be summarized as follows. First, this study was the first report on the clinical data of the rectus abdominis muscle of adult Korean women who were measured using CT, not ultrasonography. Second, pregnancy and delivery were considered to be the factors that influenced the thickness of the rectus abdominis muscle. Third, it was confirmed statistically that a previous laparotomy could have a negative influence on the upper portion of the rectus abdominis muscle. Fourth, the importance of the thickness of the rectus abdominis muscle was reinterpreted from the surgeon's perspective.
To achieve wide clinical application, a prospective study is necessary to verify the importance of the thickness of the rectus abdominis muscle in that it is a factor that predicts flap survival; such a study would be more useful to surgeons than would a study with a retrospective design.
Notes
No potential conflict of interest relevant to this article was reported.