Lymphatic vessel mapping in the upper extremities of a healthy Korean population
Article information
Abstract
Background
Intraoperative indocyanine green (ICG) lymphography can effectively detect functioning lymph vessels in edematous limbs. However, it is sometimes difficult to clearly identify their course in later-stage edematous limbs. For this reason, many surgeons rely on experience when they decide where to make the skin incision to locate the lymphatic vessels. The purpose of this study was to elucidate lymphatic vessel flow patterns in healthy upper extremities in a Korean population and to use these findings as a reference for lymphedema treatment.
Methods
ICG fluorescence lymphography was performed by injecting 1 mL of ICG into the second web space of the hand. After 4 hours, fluorescence images of lymphatic vessels were obtained with a near-infrared camera, and the lymphatic vessels were marked. Three landmarks were designated: the radial styloid process, the mid-portion of the cubital fossa, and the lower border of the deltopectoral groove. A straight line connecting the points was drawn, and the distance between the connected lines and the marked lymphatic vessels was measured at 8 points.
Results
There were 30 healthy upper extremities (15 right and 15 left). The average course of the main lymph vessels passed 26.0±11.6 mm dorsal to the styloid process, 5.7±40.7 mm medial to the mid-cubital fossa, and 31.3±26.1 mm medial to the three-quarters point of the upper landmark line.
Conclusions
The main functioning lymphatic vessel follows the course of the cephalic vein at the forearm level, crosses the mid-cubital point, and travels medially toward the mid-axilla.
INTRODUCTION
Lymphedema is a chronic disease caused by damage to the lymphatic system. It results from disruption of lymphatic circulation, leading to slow progression of fluid retention and tissue swelling [1]. Lymphatic blockage can be induced by surgery, radiation therapy, infection, or trauma [1]. Recently, lymphedema has received more attention because it is a relatively common complication resulting from the treatment of malignancies [2].
In lymphedema, structural changes in the lymphatic system occur before the appearance of distinct clinical symptoms. The morphologic changes of the lymphatic system include abnormal dilation and irregular bending of the lymphatic vessels. In addition, abnormal retention of contrast medium was observed in an imaging study [3].
The standard treatment of lymphedema includes compression garments and intensive bandaging and massage of the affected limb [2]. For patients unresponsive to conservative therapy, surgical options are available, including excisional methods, circumferential liposuction, microsurgical lymphovenous shunts, and vascularized lymph node transfer [2]. Lymphovenous anastomosis (LVA) has several advantages over other surgical procedures. It can be performed under local anesthesia, is less invasive than other surgical techniques, and is effective in severe cases refractory to conservative therapy [4-8]. In order to maximize the therapeutic effect of LVA, it is important to properly identify the lymphatic vessels and to choose an incision site for anastomosis within a limited operative time [4,8,9].
Indocyanine green (ICG) lymphography has recently been used to visualize superficial lymphatic flow. Lymphography using ICG as a tracer for the diagnosis of lymphedema is a safe, effective, and minimally invasive method of lymphatic vessel mapping. It allows surgeons to find the precise location of the lymphatic vessels for microscopic surgery [1,8,10-13]. Surgeons can obtain real-time video images by tracing ICG dye movement using a near-infrared camera [14]. However, it is sometimes difficult to clearly identify the course of lymphatic vessels in later-stage edematous limbs. As lymphedema progresses, the pattern of lymphography becomes increasingly unclear, and eventually becomes diffuse, making it more difficult to find intact lymphatic vessels for LVA in patients with late-stage lymphedema [13]. Therefore, surgeons might have to rely on their experience to decide the location of the skin incision when finding lymphatic vessels.
Large lymphatic vessels in the upper extremity travel close to the cephalic vein of the arm [15]. However, only a few studies have demonstrated the normal course of lymph vessels in the upper extremities. The purpose of this study was to elucidate the lymphatic vessel flow patterns in healthy extremities in a Korean population and to use these findings as a reference for the treatment of patients with lymphedema.
METHODS
Between March 2016 and July 2017, we conducted a study using ICG lymphography. Fifteen volunteers (12 males, 3 females) were included in this study. Overall, 30 upper extremities were evaluated (bilateral limbs of 15 healthy volunteers). The age of the volunteers ranged from 24 to 41 years (average, 28.3 years), and body mass index (BMI) ranged from 19.1 to 27.1 kg/m2 (average, 22.7 kg/m2) (Table 1). All volunteers provided written informed consent for this study.
A subcutaneous injection of 1 mL of ICG (Dongindang Pharm., Siheung, Korea) mixed with 2% lidocaine was made into the upper extremities at the second web space of the hand. After 4 hours, fluorescence images of lymphatic vessels were obtained with a near-infrared camera (Moment K; IANC&S, Seoul, Korea), and the lymphatic vessels were marked (Figs. 1, 2). Three landmarks were designated: (1) the radial styloid process, (2) the mid-portion of the cubital fossa, and (3) the lower border of the deltopectoral groove. A straight line connecting the points was drawn, and the distance between the connected lines and the marked lymphatic vessels was measured at 8 points (Fig. 3). If 2 or more lymph vessels were visible, the one with the largest diameter was chosen. If they appeared similar, the middle position was marked. All data are expressed as mean and standard deviation (Fig. 4).
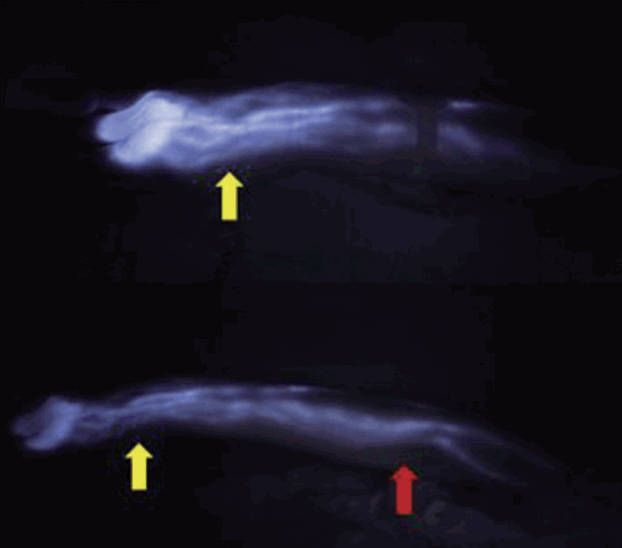
A real-time ICG fluorescence image
An ICG fluorescence image taken with a near-infrared camera 30 minutes after injection of ICG dye (top). Lymphatic flow was clearly detected in the upper arm 4 hours later (bottom). Yellow arrows indicate the wrist, and red arrow indicates the elbow. ICG, indocyanine green.
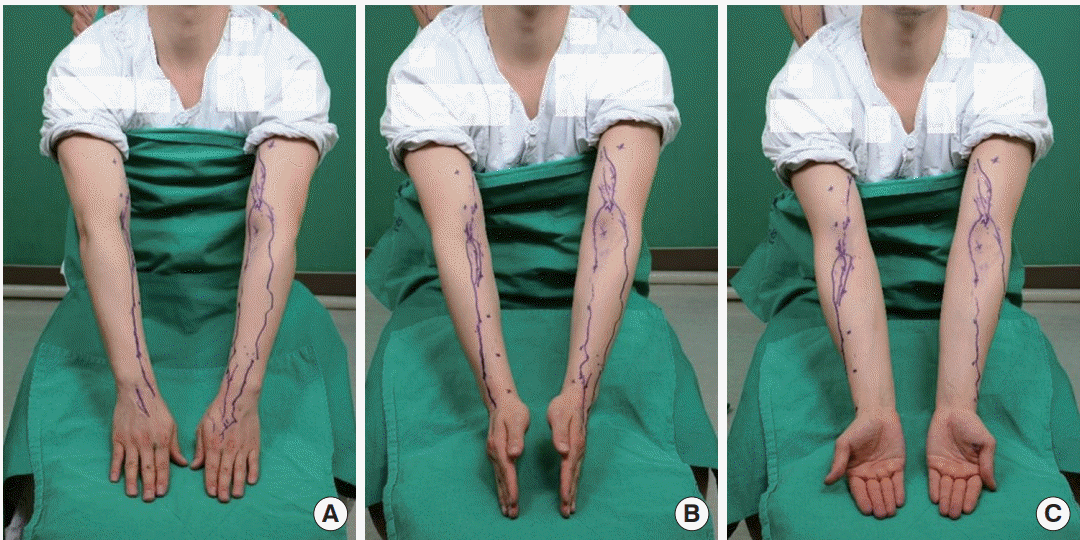
Lymphatic vessels marked on the body
Each small ‘X’ represents a landmark, and the purple line indicates lymphatic vessels detected with a near-infrared camera.
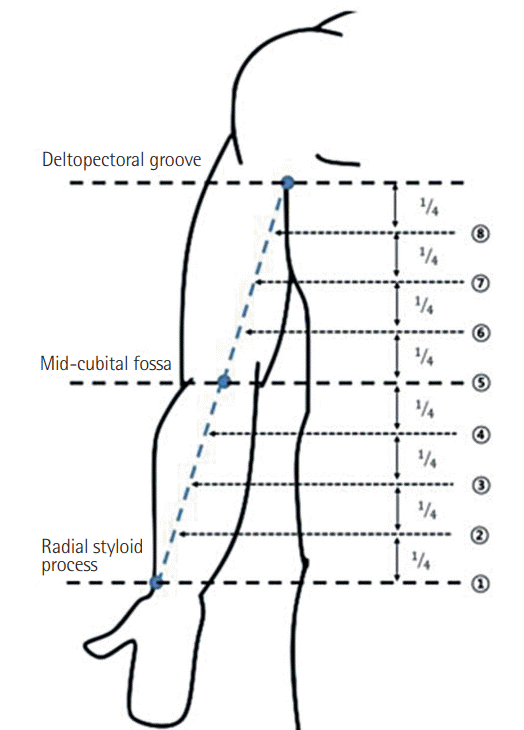
An illustration of the landmarks
A straight line connecting the points was drawn and divided into 4 equal lengths.
RESULTS
Thirty healthy upper extremities (15 left and 15 right) were included in this study. From the distal end, the lymphatic vessel ran dorsal to the styloid process, passed the midpoint of the cubital fossa, and then coursed toward the ulnar side (Fig. 2). The average course of the main lymph vessels passed 26.0±11.6 mm dorsal to the styloid process, 5.7±40.7 mm medial to the mid-cubital fossa, and 31.3±26.1 mm medial to the three-quarters point of the upper landmark line (Fig. 4). In addition, the ratio of the distance between the marked lymphatic vessels and landmarks to the circumference of each point was calculated and expressed as a percentage (Fig. 4).
DISCUSSION
Upper extremity lymphedema is a distressing complication that can occur after the treatment of breast cancer. It is often chronic in course and difficult to manage [16]. Among the several diagnostic tools for detecting lymphedema, lymphoscintigraphy is a widely available imaging test that allows the detection of abnormal lymphatic circulation and localization of the lymph nodes [13]. Unfortunately, it involves radiation exposure and the use of a radioisotope, and cannot visualize the precise course of the lymphatic vessels [13].
ICG lymphography uses a non-radioactive medium as a tracer [13]. As it does not carry the potential risk of radiation, ICG lymphography is the routine modality for evaluating lymphedema [1,10,12,14,17-19]. In 2007, Ogata et al. [1] and Unno et al. [18] reported the clinical application of ICG lymphography for the evaluation of lymphedema. Many authors have investigated the clinical applications of ICG lymphography, which currently is used to obtain important information for lymphatic functional assessment and preoperative evaluations before microsurgical procedures [1,10,14,18]. The sensitivity of lymphedema detection using ICG lymphography was almost 100% in a rat model [1]. With the proper injection of ICG into a subcutaneous layer devoid of blood vessels, the medium flows into the lymphatic system. If ICG is accidentally injected into an artery or a vein, the blood flow washes the medium away in less than a minute, so it will not affect the overall results [1].
Various treatment options exist for lymphedema. Most patients are treated conservatively with various compression therapies, including complex physical therapy, pneumatic pumps, and compressive garments [2,20]. Surgery is considered when medical therapy fails [8]. Among the many types of surgical treatments, LVA, which involves a bypass connecting the congested lymph vessels to the venous system, is the least invasive and most effective surgical treatment [8].
ICG lymphography has been widely used to visualize superficial lymphatic flow. It visualizes superficial (1–2 cm in depth) lymphatic vessels in real time, providing the exact location of lymphatic vessels for LVA surgery [1,8,10-13]. This allows surgeons to more accurately determine the skin incision site for LVA, to shorten the incision length, and to reduce the operative time [6,8].
However, ICG lymphography can barely visualize lymphatic vessels deeper than 2 cm from the skin surface and is less accurate in severe cases with the extension of dermal backflow (DB) patterns [11-13]. For patients with severe lymphedema, DB patterns exist throughout the limb, and the lymphatic vessels cannot be visualized by ICG lymphography [8]. It is also difficult for the surgeon to determine the incision site for LVA. To solve this problem, we identified the approximate location of the lymphatic vessels in the upper extremities of healthy persons for use as a reference during surgery.
In 2007, Suami et al. [15] reported that the course of superficial lymphatic vessels was often concentrated near the major draining veins, especially the cephalic and basilic veins. According to that study, all lymph vessels from the hand and the forearm flowed into the lymph nodes in the axillary region, which is consistent with our findings [15]. They also reported that lymph collecting vessels were located in the subcutaneous fat tissue, and that their diameters remained similar throughout their course [15]. In addition, Amore et al. [21] conducted an anatomical study regarding lymphatic drainage of the body. They identified the lymphatic currents of the upper limbs that form networks between each other, and run alongside the superficial veins at the forearm level, then towards the axillary lymph nodes [21]. In the studies of Suami et al. [22] and Ohtsubo et al. [23], ICG lymphography was used to observe the superficial lymph flow in the upper extremity. In those studies, lymphatic flow emerged as a linear pattern longitudinal to the medial side of the upper arm, which is similar to our results [22,23]. The significance of our study is that it is the first to demonstrate and quantify the location of functioning lymphatic vessels in the upper extremity, to the best of our knowledge.
This study has some limitations. First, the study group was relatively small, with only 30 upper extremities. Second, there were more male than female volunteers due to some hesitation regarding body exposure. Third, using ICG for lymphography requires an injection. We used 2% lidocaine with ICG to reduce the pain of injection. In addition, ICG has intrinsic side effects, such as nausea and fever, in less than 1% of patients, as described previously [1,24]. No side effects associated with ICG were documented in our study. Fourth, micro-lymphatic vessels could not be detected in our study. In the study conducted by Ogata et al. [1], the diameter of the lymph vessels detected using a near-infrared camera was more than 0.1 mm, which was similar to our results. However, the diameter of the lymph vessels was sufficient for the purpose of our study, which was to identify appropriate surgical incision sites. Recent studies have shown that lymphatic vessels between 0.3 mm and 0.6 mm in diameter are used in LVA [6]. According to a study by Yamamoto et al. [8], LVA was successfully performed in a 0.45-mm-diameter lymphatic vessel. Fifth, our technique is reliable only for the lymph vessels in the dermis and subcutaneous fat layer due to the detection depth of the infrared camera system. Therefore, the deep lymphatic system could not be assessed in our study. However, the superficial lymphatics in healthy individuals or patients with early stage of lymphedema are usually maintained. Thus, they might be more important for lymphatic drainage than the deep lymphatic chains [14].
Finally, further studies are needed to apply our results to actual treatment. In our study, the distance between the lymphatic vessel and the landmark was divided by the circumference and expressed as a percentage, to account for physical differences among the subjects. The results of our study might have useful clinical applications for surgeons when predicting the location of lymphatic vessels for LVA.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB No. AS17191) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.