Management of Malignant Melanoma
Article information
ONCOLOGIC BACKGROUND
Incidence
Malignant melanoma is the leading cause of death in skin cancers. The incidence of melanoma is reported to vary throughout the world. It demonstrates a clear demographic disparity, and is a common malignancy in Western countries. It is reported at the highest rates in Auckland, New Zealand, with the age-standardized rate of 40.2/100,000 [1]. Other high rates have been reported in Australia, with a rate of 37.7/100,000 among males and 29.4/100,000 among females [2]. In the United States, 68,720 patients diagnosed with and 8,650 deaths from malignant melanoma were reported in 2009 [3]. In Asia, malignant melanoma is a rare disease and it is difficult to locate reports on the incidence. The age-adjusted rate for melanoma in 2006 was known to be 0.65/100,000 and 0.71/100,000 for males and 0.59/100,000 for females in Taiwan. To the best of our knowledge, there has been no multi-center study of malignant melanoma in Korea.
Etiology
UV exposure has been implicated as a major cause in the etiology of malignant melanoma [4]. However, acral lentiginous melanoma (ALM) and mucosal melanoma, which are more common in Asia, are generally not a result of exposure to ultraviolet irradiation. The etiology is yet to be determined. In an epidemiologic study of ALM from Australia, an increased risk was associated with penetrating injury of the feet or hands (relative risk [RR], 5.0) and with heavy exposure to agrichemicals (RR, 3.6) [5]. Further etiologic studies to identify the risk factors are warranted.
MORPHOLOGIC SUBTYPE
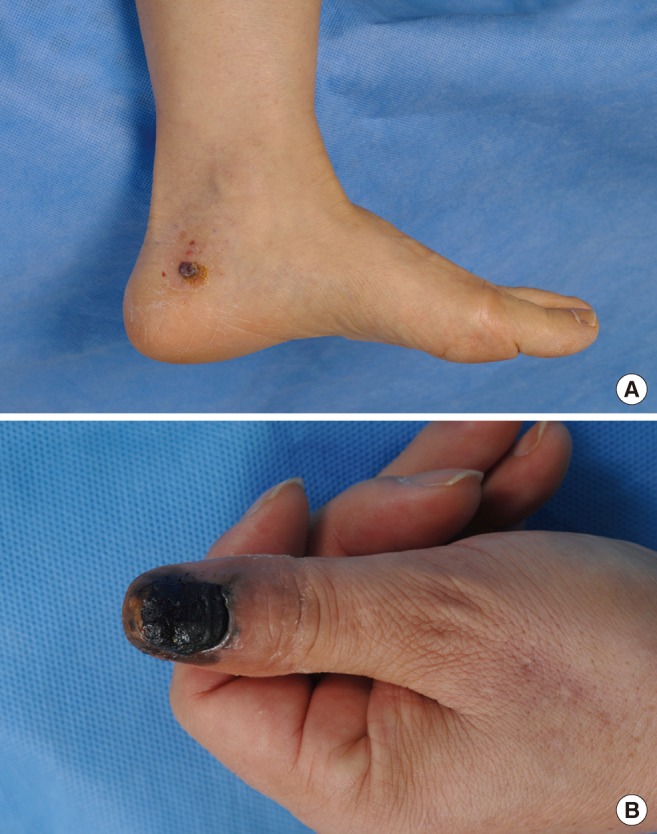
The cardinal clinical feature of malignant melanoma is a pigmented lesion that changes visibly over a period of months to years. Any lesion noted to have changed in color, shape, size, or elevation warrants medical attention. Changes in lesions are highlighted in the 'ABCDE Guidelines' (Asymmetry, Border irregularity, Color change, Diameter >6 mm, and Evolution [changing lesion] or Elevation; previously 'ABCD Criteria') [6]. The letter 'E' for evolution highlights the importance of changing lesion in appearance, size, shape, color, and symptoms. The major growth patterns of melanoma are lentigo maligna (LMN), superficial spreading (SMM), nodular melanoma (NM), and ALM [6] (Fig. 1). The most common subtype in Caucasians is SMM, which has a good prognosis. However, in Asians, ALM is the most common subtype, and it is diagnosed in the advanced stage.
STAGING
Biopsy
Adequate biopsy is the first step for a definite diagnosis of malignant melanoma. Whether cutting into a tumor leads to a poor prognosis for the patient has been debated for decades. The a noli me tantere ("do not touch me") strategy is considered appropriate. There is still concern that incomplete biopsy or punch biopsies will harm, although several studies have shown no negative influence on patient survival [7-12]. However, incisional biopsies do not seem to influence patient prognosis. Nevertheless, a complete excisional biopsy should generally be performed whenever possible. For a lesion clinically suspicious for malignant melanoma, one should perform an excisional biopsy with a clinically negative margin when possible to a full depth sufficient to ensure that the lesion is not transected [13-19]. It has been recommended that 1 to 3 mm margins are required to include the subclinical component of most atypical melanocytic lesions [20]. A number of biopsy methods have been known for a diagnosis of malignant melanoma including elliptical punch excision, or shave removal to a depth below the anticipated plane [17]. Shave biopsy may compromise pathologic diagnosis and make it difficult to measure the thickness. Clinically clear margins on excisional biopsy that encompass the entire width are preferred. However, incisional biopsy of the most atypical portion of the lesion is an acceptable technique in select circumstances, such as a facial or acral lentiginous lesions, or those with low clinical suspicion or very large lesions for which is impossible to obtain the whole lesion [14]. If an incisional biopsy specimen is inadequate for definite diagnosis of melanoma, a repeat biopsy should be performed [14].
In a suspicious nail lesion, the nail matrix should be sampled. Because of anatomical complexity of the nail area and the fact that melanoma arises in the nail matrix, suspicious nail lesions are best evaluated in the nail apparatus. For suspicious subungal lesions, the nail plate should be sufficiently removed to expose the underlying lesion and a biopsy should be performed with an adequate amount of atypical tissue.
2010 American Joint Committee on Cancer TNM definitions
A new American Joint Committee on Cancer (AJCC) cutaneous malignant melanoma staging system was published in 2010 [21]. This revised system was developed on the basis of a multivariate analysis of 30,946 patients with stage I, II, and III melanoma and 72,972 patients with stage IV melanoma. New findings include the following: 1) In patients with localized melanoma, tumor thickness, mitotic rate (mitoses/mm2), and ulceration were the most powerful prognostic factors. 2) Mitotic rate replaces level of invasion as a primary criterion for T1b melanoma. 3) Among the 3,307 patients with regional metastases, 'the N components' were defined as the tumor burden, the ulceration of the primary lesions, and the number of metastatic nodes. 4) All patients who have microscopic nodal metastases, regardless of extent of the tumor burden, are classified as stage III. Micrometastases can be detected by immunohistochemistry. 5) On the basis of analysis of patients with distant metastases, the two dominant components were included in defining of the 'M component': Site of metastases and an elevated serum lactate dehydrogenase level.
TREATMENT
Surgery is the most important modality for malignant melanoma. Surgery means wide excision of the primary site, surgical management of clinically normal and abnormal lymph nodes, and surgery for distant metastases.
Wide excision of primary melanoma
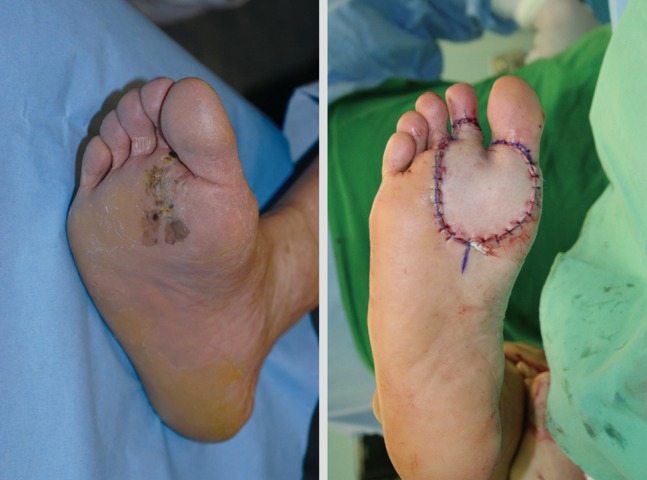
The primary treatment modality for malignant melanoma is surgical excision (Fig. 2). After the diagnosis of melanoma has been histologically confirmed and the primary lesion has been adequately staged, a wider and deeper excision is needed. It is reported that melanoma cells may extend micro-satellite fashion from several millimeters to several centimeters from the clinically visible lesion.
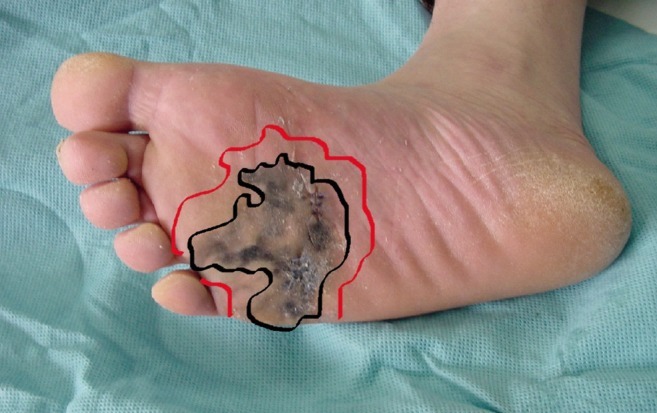
Wide excision of malignant melanoma of the plantar area (black line, margin of tumor; red line, margin of wide excision).
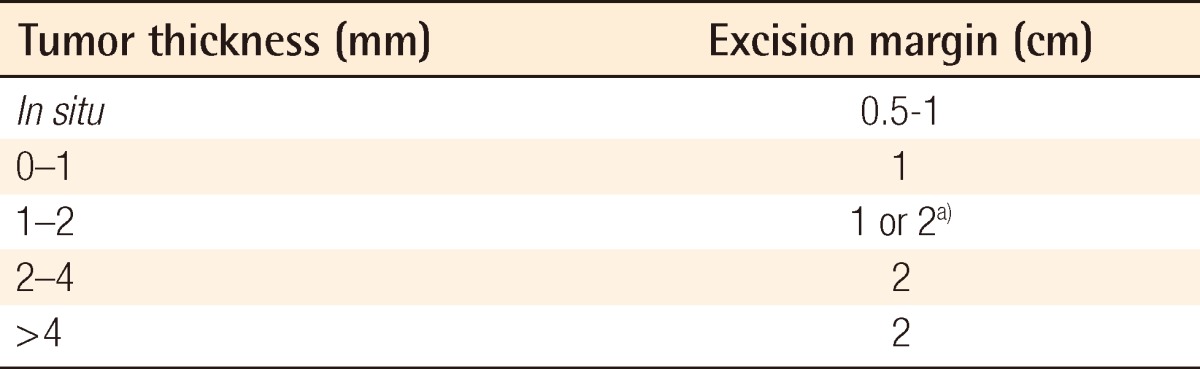
Excision margins for melanoma have been a major subject of heated debate in recent years. Recommended surgical margins are based on a prospective randomized controlled study for survival and mortality (Table 1). The primary goal of wide excision of malignant melanoma is to achieve a histologically negative margin and prevent local recurrence. Surgical margin recommendations are based on studies in which margins were clinically measured around the primary tumor and including these basic concepts: 1) Wide excision is associated with a reduced risk of local recurrence; 2) there is no evidence in thin melanoma (<1 mm) to confirm the improvement of the survival or local recurrence rate with excision margins exceeding 1 cm; 3) Excision with greater than 2-cm margins offers no benefit in the survival rate or local recurrence [22].
Melanoma of 1.0 mm or less (T1). Wide excision with a 1.0 cm margin is recommended. However, many surgeons consider 0.5 cm margins the standard of care for excision of melanoma in situ. For melanoma between the depth of 1.0 and 2.0 mm, 1 to 2 cm margins are adequate while 2 cm margins are adequate for lesions up to 4 mm thickness (Fig. 3). Although patients with lesions above 4 mm in thickness have a relatively high risk of local recurrence, there are few data to support the use of margins wider than 2 cm [23]. In desmoplastic melanoma, which shows locally aggressive behavior, a resection margin of 3 to 5 cm is needed [23].
Excision margins should be performed with known guidelines, but may involve amputation depending on the anatomical location of the lesion. For more complex areas, such as the perineum, fingers and toes, or where the primary melanoma involves the anatomic areas not amenable to simple wide excision, a multidisciplinary treatment modality should be sought. Caution should be exercised in choosing the width of the margins of in situ melanomas as there are no known randomized studies.
Regarding excision depth, the recommended depth for invasive melanoma has always been to the level of the muscle fascia; no unequivocal evidence exists to support that this is necessary in every circumstance such as in anatomic locations with an a thick fat layer. Although no recommended depth of excision exists, the expert group recommends that, whenever possible, excision should be performed down to the muscle fascia, or at least deep adipose tissue [20,24].
Special areas
Scalp
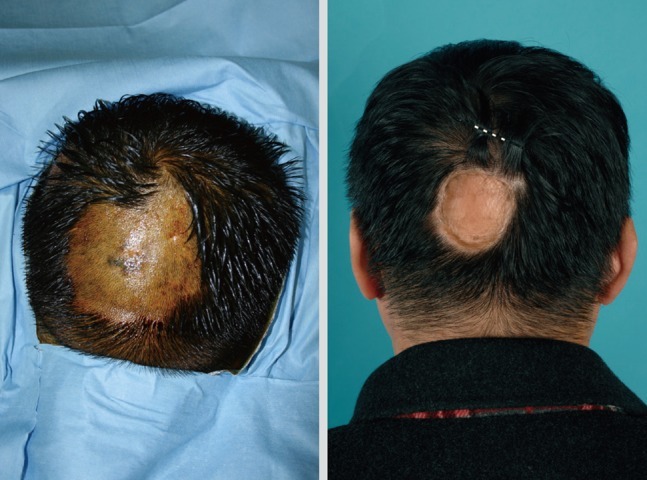
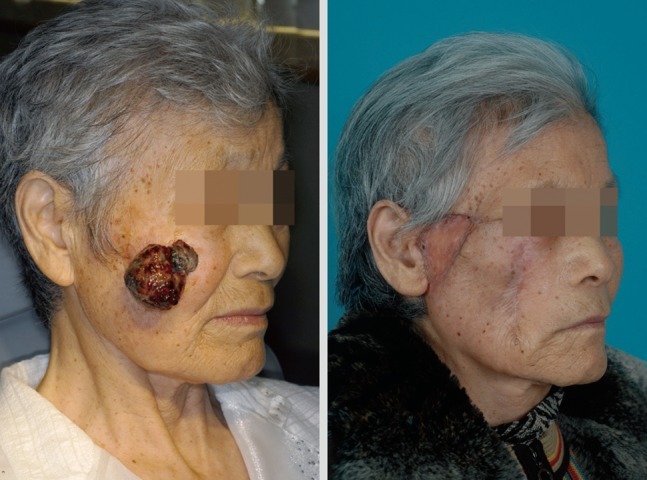
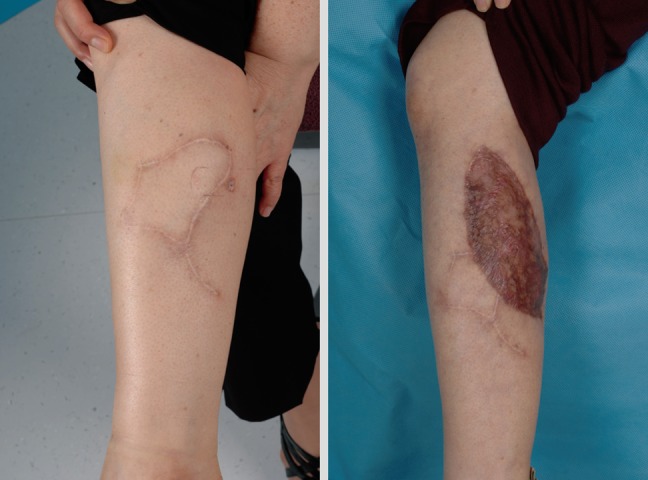
Deciding on the surgical management of the scalp area is challenging because reconstructive options are limited. Even after free flap surgery reconstruction, an additional operation will be needed for resolving the alopecia. Some authors recommend that scalp melanoma should be excised including the 'galea aponeurosis' down through the pericranium. In this clinical situation, reconstruction is difficult because a skin graft is impossible and local flap usage is not desirable due to the risk of recurrence (Fig. 4). Also, malignant melanoma in the head and neck area shows a high mortality compared with other sites, when controlled for other factors [25].
Ear and face
Wide excision of the lesions in the face is limited to avoid injury or deformity of vital structures which is important for cosmetic results (Fig. 5). The ear is the site of primary malignant melanoma in 7% to 20% of cases of head and neck melanoma [26]. Depending on the lesion in the ear, in most cases, it is best treated with wedge resection. When the excision has been performed with an initially narrower margin, the surgeon should obtain a negative resection margin. The excision depth should be down to the perichondrium and include the cartilage. Partial or total auriculectomy should be performed in only recurrent or extensive disease. Lymphatic drainage of the ear is to flow to the parotid basin and the superficial parotidectomy and anterior neck dissections are indicated in positive sentinel lymph nodes. The drainage is occasionally into the posterior neck, and level V lymph node dissection should be indicated.
Digit
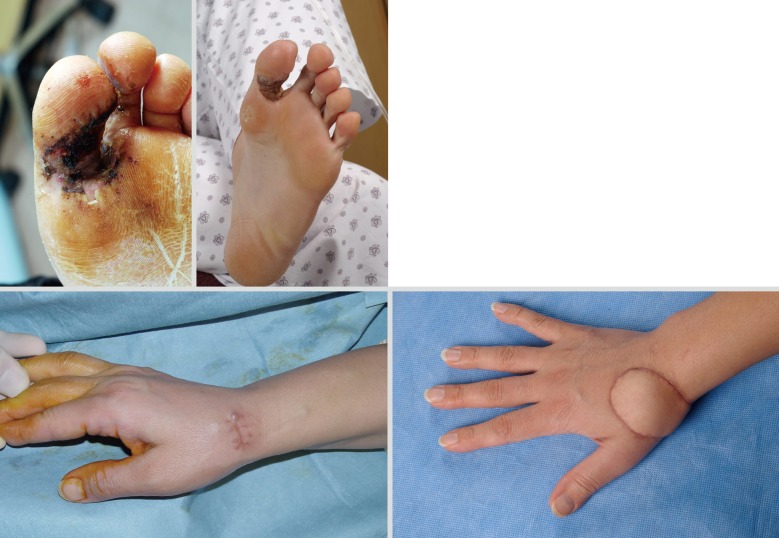
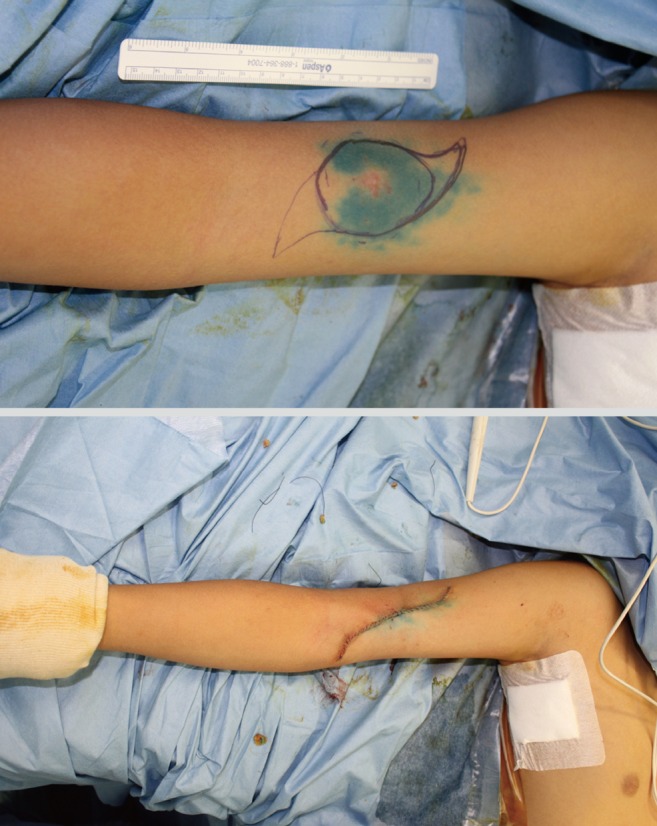
Excision of melanoma of the toe or finger is controversial. The traditional method of treatment is amputation of the involved digit. The thumb is the most commonly affected digit in the hand, and loss of its function can be devastating. In primary lesions of the subungal or distal phalanx, the digit should preferentially be amputated at the level proximal to the distal interphalangeal (DIP) joint to preserve as much of the length of the digit as possible (Fig. 6). Kozlow and Rees [27] insisted that superficial lesions (below 1 mm) could be treated with wide excision. Only in invasive lesions (greater than 1 mm), is amputation of the digit required at one joint proximal to the lesion. For the index finger, ray amputation should be preferred considering the improved functional ability in pinching.
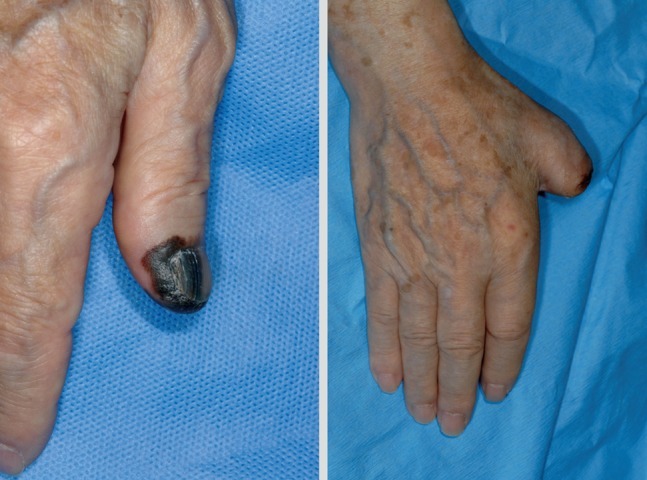
Male 76-year-old, acral lentiginous malignant melanoma with amputation just proximal to the distal interphalangeal joint.
Recent studies have shown that more distal levels of amputation do not compromise the survival or recurrence rate [28]. Unless the bone is directly involved, total amputation of a finger or ray amputation is not mandated for proximal finger lesions. Also, the digit-sparing approach shows good results [28]. Adequate soft tissue excision with reconstruction should be performed to prevent functional impairment.
Sentinel lymph node biopsy and lymphadectomy
The initial site of metastasis in melanoma is via the lymphatics in the majority of cases [29]. In general, nodal metastases in melanoma happen in an ordered manner. Involvement of the sentinel lymph node (SLN) occurs first, then the more distal lymph node (LN) is involved. The incidence of skip metastasis (cases of negative SLN with positive involvement of non-sentinel LN) is <5% [30,31]. Lymphatic mapping using lymphoscintigraphy and intraoperative injection of radioisotope and/or blue dye is used to identify the lymph node immediately flown from the primary lesion [30,32,33]. Histologic examination of the sentinel lymph node is known to be the most important prognostic indicator for disease-specific survival of patients with melanoma greater than 1 mm in thickness (T2) [34-36]. The overall rate of SLN positivity among patients with intermediate depth melanoma is approximately 15% to 20%, and it significantly decreases in melanoma with less than a 1 mm thickness [37-39]. Sentinel lymph node biopsy should be considered in patients with melanoma >1 mm in thickness (T2, 3, and 4), and it is not recommended for patients with melanoma in situ or T1a lesions. In patients with T1b melanoma of 0.76 to 1.00 mm thickness, sentinel lymph node biopsy should be discussed; in T1b melanoma, with tumor thickness <0.75 mm, sentinel lymph node biopsy should not be considered, unless other adverse parameters in addition to ulceration or increased mitotic rate are present, such as angiolymphatic invasion, positive deep margin, or young age [20]. Some authors recommend sentinel lymph node biopsy for most patients with melanomas >0.76 mm and increased mitotic rate [40].
SPECIAL CONSIDERATION
Surgery in advanced malignant melanoma
Surgical excision of metastatic lesions can increase the survival rate in selected patients. The median survival for a metastatic melanoma in one site is 7 months, for a patient with disease at 2 sites is 4 months, and when 3 sites are involved, the median survival is 2 months. The numbers of metastases is the most significant factor predicting survival, and the location of metastases is also important [41].
The prognosis of AJCC stage IV melanoma is known to be less than 10% in 5 years [42] and a multimodal treatment method including chemotherapy, radiation therapy, and limb-infusion therapy is usually offered. However, surgery is the most powerful modality for palliation and prolonging life. Decision making regarding surgery of advanced lesions should take into consideration multiple factors including the number of metastases, rate of growth, types of and responses to previous treatment, and the age, medical condition, and desires of the patient [43].
Surgery should be chosen when resection is possible in a case of a lesion that is clinically isolated, and especially in skin and soft tissue lesions, complete resection with an adequate resection margin should be performed (Fig. 7). Multiple resections also are possible. However, cure is usually not a realistic aim, and treatment must reflect a carefully considered judgment concerning attempts to improve or preserve the quality of life.
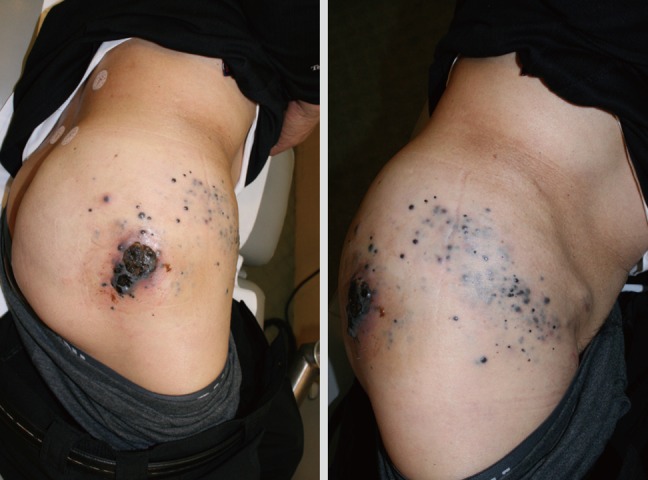
In-transit metastasis
In-transit metastasis means the clinical manifestation of small tumor emboli trapped within the dermal and subdermal lymphatics between the site of the primary lesion and regional lymph node drainage basin (Fig. 8) [44]. The choice of therapy depends on the number of lesions, their anatomic location, their depth and size, and the presence or absence of extraregional disease. Their treatment options are classified into local, regional, and systemic therapy [20]. Surgical excision is appropriate as an initial treatment, and it should be performed until the negative margin (Fig. 9). Some authors recommend the wide excision with a 2 cm margin [24]. However, surgical excision alone often fails to control regional disease. Dong et al. [45] reported that the patients who showed the recurrences after wide excision developed the locoregional recurrences were seen in 55% of patients by 2 years and in 82% by 5 years. Also, intralesional injection of Bacillus Calmette-Guérin (BCG), dinitrochlorobenzene, or interferon has been reported [46]. In regional therapy, regional infusion or perfusion chemotherapy has been performed. However, in patients with recurrent in-transit metastasis who are not candidates for either local or regional therapy, systemic therapy such as chemotherapy or immunotherapy should be considered [47]. Most patients who show 'in-transit metastasis' will die of their disease. The factor most predictive of a poor outcome was revealed to be the presence of subcutaneous metastasis (as opposed to dermal-only metastasis) [48]. Also, the most important predictor of survival is the type of recurrence, with patients of distant metastasis having a 5-year survival of 10% [49].
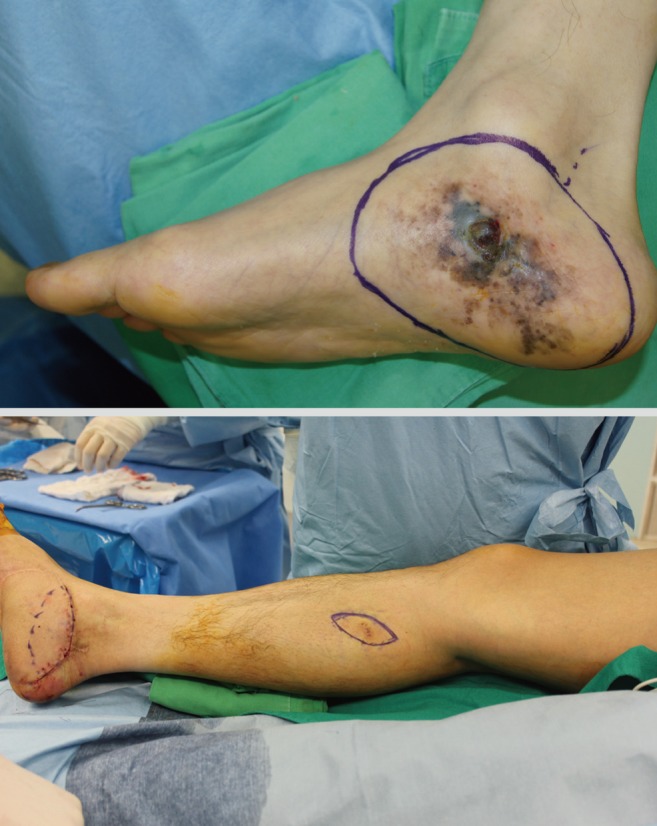
Local recurrent disease
Local recurrence means a regrowth of melanoma within 2 cm of the primary surgical excision scar [50]. Most recurrences occur in the first 2 years and greater tumor thickness is associated with an increased risk of local recurrence [51]. Also, incidence of local recurrence increases with the presence of ulceration and location of the head, neck, or distal extremity. Recurrence after 10 years is infrequent (below 3%) [52]. Median time intervals and ranges between the initial visit and diagnosis of recurrences are reported according to the AJCC staging. Stage 1 had a median recurrence of 22 months (range, 2.0 to 60.5 months) and median recurrence of stage 2 is 13.2 months (range, 2.0 to 71.0 months). In stage 3, median recurrence is known to be 10.6 months (range, 2.3 to 53.8 months). Local recurrence is closely related to high mortality. Surgeons should concentrate their efforts on excising enough tissue for preventing repeated recurrence. Surgery is also the mainstay of treatment (Fig. 10). Although a 5-cm margin was once recommended, recent clinical trials have demonstrated that narrower margins lead to equivalent local control [53]. In the absence of significant data, some authors recommend excision using approximately 1 cm margins. However, few data exist about appropriate surgical margins because follow-up is quite short in these patient groups. After the surgery, high dose interferon alpha 2b is sometimes considered.
Children and young people
Melanoma accounts for 1% to 3% of older childhood malignancies [54]. The incidence of melanoma in childhood is strongly related to age and sex. Melanoma is exceedingly rare in children younger than 10 years [55]. A sharp increase occurs in puberty and is more pronounced in girls. In patients diagnosed with melanoma, approximately 50% have regional disease after sentinel lymph node biopsy or radiologic examination [54]. In pediatric patients, amelanotic melanoma is shown more frequently, which delays definite treatment. Unlike the adult population, it is unclear whether a negative sentinel lymph node biopsy is a prognostic indicator for survival [56]. Sentinel lymph node positivity rates are reported to be higher in children than adults (44% vs. 23%) [57]. Disease-free survival and overall survival seem to be similar to the rates for melanoma of adults, and sentinel lymph node biopsy has been shown to be safe [54].
For melanoma in childhood, as in adults, surgery is the primary modality of treatment. Wide local excision is recommended to be practiced with the same margin guidelines as those for adults (Fig. 11). Also, adjuvant therapy such as interferon alpha 2b is more tolerable in children than adults [58]. However, melanoma in childhood is very rare and limited data exist for evaluating the outcomes.
Notes
The author thanks Bo Young Park, MD and So-Young Lim, MD, PhD for their contribution in preparing this article.
No potential conflict of interest relevant to this article was reported.