Smile Restoration for Permanent Facial Paralysis
Article information
LEARNING OBJECTIVES
After reviewing this article on facial paralysis, the reader should be able to do the following:
1) Review the epidemiology and causes of facial paralysis;
2) Describe the assessment of a patient;
3) Describe the treatment in adults and children; and
4) Understand the outcomes of facial reanimation surgery.
INTRODUCTION
The loss of facial expression and the disfigurement of facial paralysis have serious implications for a patient's physical and psychological well being [1]. Numerous aetiologies of facial paralysis exist but once nerve recovery has been static for two years, interventional surgery is required to improve the situation [2]. Facial paralysis is often treated as an aesthetic problem but can also have real physical and psychological problems. These include difficulty with speech, low self-esteem, poor social interaction, oral incontinence, and dental problems; caries may develop due to the lack of food progression through the oral cavity and repeated trauma and ulceration caused by biting of the inside of the paralysed cheek. Poor understanding of the treatment modalities available and an element of 'postcode lottery' have an impact on the service a patient may receive in the UK. This was highlighted in a recent communication from the British Association of Plastic and Reconstructive Surgery (BAPRAS) Facial Palsy Interest Group.
EPIDEMIOLOGY
Facial palsy is a condition that affects the facial nerve, one of twelve cranial nerves. Its main function is to control all 17 muscles of facial expression [3]. This involves the ability to express emotion by controlling the position of the mouth, eyebrows, and nostrils, and control eye closure, drooling, speech, and dental hygiene. A spontaneous facial palsy can present at any age but is most frequently seen at age 20 to 50 years, affecting both sexes equally. Incidence is around 30 cases per 100,000 per year and is slightly higher in pregnant women (45 per 100,000) [4] and an incidence of 1.8 per 1,000 children are born with facial palsy [5].
CAUSES
There are discreet patient groups that can best be broadly classified into children and adults; this is the most important criteria for determining the treatment options available. In children, the majority of cases of facial palsy are of an idiopathic aetiology; however, congenital and obstetric injuries are also important causes. In congenital cases, it is important to ascertain whether the palsy is unilateral or bilateral and if the palsy is part of a larger syndrome, for example in Möbius syndrome or CHARGE syndrome.
In adults, Bell's palsy is by far the most common cause. This is typically transient and spontaneously recovers; however, a small proportion will have persistent palsy to varying degrees. Direct trauma to the facial nerve and neoplasia are the other main causes. During parotid surgery, the facial nerve is particularly vulnerable and may also be disrupted by parotid gland tumours or acoustic neuromas. In rare cases, iatrogenic injury can be incurred during other types of surgery.
ASSESSMENT
A detailed history and thorough clinical neurological examination must be performed. In children, a detailed obstetric history is important, identifying concerns the parents have regarding the child and determining any functional problems that may be present. Examining the child may be difficult, but an overall impression can be made of the static position of the face at rest and when the child is smiling. It is crucial to determine if the contralateral side is also functioning normally.
Performing a full neurological assessment in adults is somewhat easier. Again, assessing the position of the face at rest and whilst smiling is important. Examination of the main branches of the facial nerve is obligatory, and it is also prudent to determine the functioning of the trigeminal and hypoglossal nerves; these are important surgical 'donor' nerves, which may be used in future surgical procedures. The remaining cranial nerves should be assessed, especially if there is concern that the palsy is part of a congenital syndrome. A full medical history is mandatory, as medical co-morbidities may impact on the available treatment options. The most useful form of investigation for assessing the function of the facial nerve or any possible donor nerves is electromyography (EMG).
It is also prudent to assess a patient's preoperative psychological status. This can initially be part of the consultation process but formal psychological referral may play a role in the care pathway of the patient [1]. This helps to manage the expectations of the patient and address what the outcome may be. It is important to provide a support network before, during, and after surgery, which potentially may be over a significant period of life. The parents of affected children often require reassurance, and this provides the ideal setting for an open discussion and prepares the family for the surgical process.
The aetiology may influence the choice of reconstruction, and generally the most important factors depend on the degree of smile impairment and the general condition of the patient. The desire to regain active motion peri-orally versus just improving the static position of the mouth will influence the choice of surgery.
TREATMENT IN CHILDREN
Since the first description of a vascularised, innervated functional muscle transfer by Harii [6] in 1976, a two-stage reconstruction has become the gold standard, especially in children [7]. In the first stage, a cross facial nerve graft is connected to a functioning terminal branch of the facial nerve, typically a buccal branch, on the unaffected side and tunnelled subcutaneously under the upper lip to the affected side (Fig. 1A). The donor nerve ideally should leave a minimal deficit; therefore, a pure sensory nerve is used, usually the sural nerve from the leg. This leaves a small patch of numbness on the outside of the ankle and foot. The nerve graft is then allowed to regenerate across the face, which normally takes around 3 to 6 months and is monitored clinically by Tinel's sign. This may be unreliable in young children, as they may not fully understand the test. In addition, Tinel's sign is a more sensitive indicator of sensory nerve than motor nerve regrowth; therefore, it is important educate the parents that this is not necessarily a sign of surgical failure. In the second stage of the surgery, the patient then receives a vascularised muscle flap that will be innervated by the cross facial nerve graft over a 6 to 12 month period (Fig. 1B). The typical muscles used are the pectoralis minor, gracilis, and latissimus dorsi, as these leave as little donor morbidity as possible. At this stage, the patient will slowly regain movement on the affected side of the face [8-10]. In bilateral congenital facial paralysis, the facial nerve is not available as a motor to drive the new muscle; therefore, another branch of a cranial nerve may be used, such as the V, IX, or XII [11]. Neurophysiological studies (e.g., EMG) play an important role in cases with bilateral facial palsy as multiple cranial nerves may be involved as part of a syndrome [12,13].
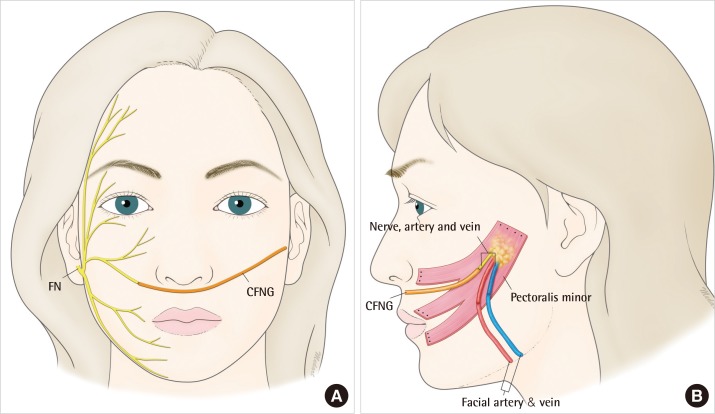
Stage 1 and 2 facial reanimation. (A) A cross facial nerve graft (CFNG), usually a harvested sural nerve, is joined to a functioning buccal branch of the facial nerve (FN) on the contralateral side and tunnelled subcutaneous to the preauricular area on the affected side. The nerve regeneration can be monitored clinically using Tinel's sign. (B) A free functional pectoralis minor flap is raised through an axillary approach with its neurovascular pedicle. The flap is inset on the affected side where the thoraco-acromial artery and vein are anastomosed to the facial artery and vein, and the medial pectoral nerve is connected to the CFNG. Reinnervation of the muscle typically takes 6 months, at which stage the muscle can be seen to start twitching.
When considering facial reanimation in children, it is important to consider the impact the surgery and hospital appointments will have on the family and the child. The average time taken to complete the treatment is around two years, and this should ideally be completed before educational commitments become affected and before the child becomes fully aware of his or her palsy and the psychological implications begin to take hold. Surgery can be started from the age of four years, and it is beneficial to allow time for the family to consider the surgical options in detail; therefore, early referral is prudent.
TREATMENT IN ADULTS
The nerve regeneration potential of a patient is inversely proportional to their age; the older the patient, the less likely they are to experience a good result from nerve grafting. It is currently accepted that one-stage reconstruction yields the most favourable results in the older age group [14]. A muscle flap with a long nerve is used to directly reach the opposite side of the face and allow the surgeon to do a direct neurorrhaphy (i.e., joining two nerves together) to a very distal branch of nerve VII of the working side of the face [15]. After the age of 60, it is probably prudent to choose the shortest innervation route of the functional muscle transfer by joining the nerve of the muscle to a branch of ipsilateral cranial nerve V, most commonly to a branch of the masseteric nerve; this gives the patient the best possibility, even with slow nerve regeneration, of gaining some active motion [16]. It is important to realize that this will require a certain amount of re-learning or 'plasticity' in order to smile spontaneously.
There are other treatment modalities available, which can be broadly grouped into static or dynamic options. Simple unilateral skin tightening procedures can be performed, but these are normally of little long-term value; the lack of muscle activity as well as the elasticity of the skin cannot sustain a corrective result. More permanent sling procedures offer an improved static position of the mouth [17]. In this type of surgery, a portion of the tensor fascia lata is harvested and inset in a fixed position to give the face a more symmetrical posture at rest. More complex local muscle transfers exist, such as the Labbe temporalis slide [18,19], the Gilles conventional temporalis transfer, and the masseteric transfer [20]. It is often difficult for patients to learn how to effectively utilize these muscles, as the plasticity of the brain also decreases with age and often multiple revisions are required over time [21].
TREATMENT OPTION WITH INCOMPLETE PARALYSIS
The most common isolated branch palsy is that of the marginal mandibular branch; this results in uncontrolled excessive retraction of the lower lip on the normal side, as it is unopposed. There are both medical and surgical treatments. Botulinum toxin A (Botox) can be injected into the healthy side of the face every 3 to 6 months in order to restore symmetry. Botox may also be used to improve involuntary twitching and fasciculation or synkinesis, which is often experienced during a patient's recovery from a Bell's palsy, but it may also be a permanent symptom [22]. Equally, the healthy muscle can be resected to improve posture [23-25].
The position of the lower lip can be corrected with a local muscle transfer of the anterior belly of the digastrics, as described by Conley [23] in 1982. Various other midface techniques such as the suborbicularis oculi fat (SOOF) lift can be used to correct the minimally displaced corner of the mouth of the affected side [26-28]. Small adjustments around the nasolabial fold or the corner of the mouth may also improve symmetry.
LONG-TERM OUTCOMES OF REANIMATION SURGERY
Various methods have been used to judge the outcomes of reanimation surgery, and opinions differ on the matter. Most authors agree that video assessment and panel judgment is important [9,10,29-32]; however, multiple outcome scales have been proposed [33]. All surgery involves a certain amount of scarring, and for patients who suffer from problematic wound healing, such as with hypertrophic or keloid scarring, the cosmetic outcome may be a disaster. The most important outcome is patient satisfaction following surgical intervention, as it is not uncommon for perspectives to differ between surgeons and their patients [34].
Bulkiness and asymmetry in the shape and volume of the paralyzed cheek compared to the unaffected side is almost an inevitable consequence of the disease and surgery. The results of surgery are not entirely predictable and may require some revision surgery over time [35-37]. Perfection after reanimation surgery is only achieved in approximately 60% of cases [38] (Fig. 2).
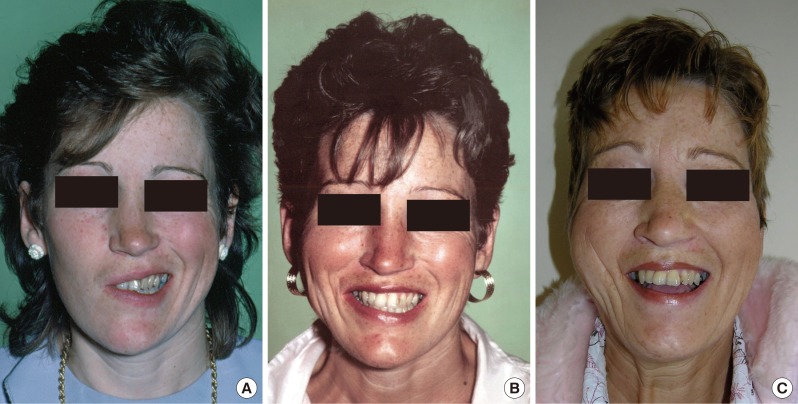
A patient following facial reanimation. (A) A patient with an incomplete right-sided facial paralysis caused by an unresolved Bell's palsy. Predominantly, the buccal and mandibular branches were affected the most, leading to the loss of smile and symmetry of the mid- and lower face. (B) Two years after completion of facial reanimation, the patient shows good excursion of the modiolus of the mouth and symmetry of the mid- and lower face. There is minimal bulkiness of the right side of the face, and the position of the mouth is restored to being symmetrical. (C) Twenty years following surgery, the patient continues to have a functioning muscle transfer with no loss in excursion or symmetry.
CONCLUSIONS
There are multiple treatment modalities available to offer an individual with facial paralysis. The delivery of these is dependent on patient awareness and primary care physician education to ensure early referral to specialist units. The treatment methods of children and adults with facial paralysis follow different algorithms based on similar principles and must be considered separately.
The potential to achieve an excellent outcome is achievable in 60% of cases. Further research has to be conducted to improve the understanding of what factors regulate nerve regeneration potential and push the balance of patients achieving an excellent result towards 100%.
Acknowledgements
The author thanks Bo Young Park, MD and So-Young Lim, MD, PhD for their contribution in preparing this article.
Notes
No potential conflict of interest relevant to this article was reported.
