A Clinical Anatomic Study of Internal Mammary Perforators as Recipient Vessels for Breast Reconstruction
Article information
Abstract
Background
Partially resecting ribs of the recipient site to facilitate easy anastomosis of the internal mammary vessels to free flaps during breast reconstruction can cause chest wall pain or deformities. To avoid this, the intercostal perforating branches of the internal mammary vessels can be used for anastomosis. The purpose of this study was to investigate the location and size of the internal mammary perforator vessels based on clinical intraoperative findings and to determine their reliability as recipient vessels for breast reconstruction with microsurgical free tissue transfer.
Methods
Twelve patients were preoperatively screened for the presence of internal mammary perforators using Doppler tracing. After modified radical mastectomy was performed by a general surgeon, the location and size of the internal mammary perforator vessels were microscopically investigated. The external diameter was examined using a vessel-measuring gauge from a mechanical coupling device, and the distance from the mid-sternal line to the perforator was also measured.
Results
The largest arterial perforator averaged 1.5 mm, and the largest venous perforator averaged 2.2 mm. Perforators emerging from the second intercostal space had the largest average external diameter; the second intercostal space also had the largest number of perforators arising from it. The average distance from the mid-sternal line to the perforator was 20.2 mm.
Conclusions
Internal mammary perforators presented consistent and reliable anatomy in this study. Based on these results, the internal mammary perforators appear to have a suitable diameter for microvascular anastomosis and should be considered as an alternative recipient vessel to the internal mammary vessel.
INTRODUCTION
The selection of suitable recipient-site vessels in autologous breast reconstruction is crucial for good surgical outcomes [1]. The thoracodorsal vessels are the most frequently used recipient pedicles in immediate breast reconstructions and are usually exposed after axillary dissection for the mastectomy procedure. However, using the thoracodorsal artery for delayed reconstruction has two disadvantages: it requires a prolonged operation time, and the dissection is difficult due to the scar from the previous mastectomy operation. For these reasons, internal mammary vessels are becoming an acceptable alternative to thoracodorsal vessels. Internal mammary vessels can be found at the third intercostal space, making their location reliably adequate for anastomosis in free flap breast reconstruction. However, the use of internal mammary vessels is also associated with the following disadvantages: chest wall contour deformity may occur due to the excision of a section of costal cartilage for vessel anastomosis, restriction of chest wall motion can arise after incising the pectoralis major muscle, and, after this surgical procedure, the internal mammary vessels cannot be used for coronary artery bypass graft surgery if required in the future [2-4].
In recent studies, using the internal mammary perforators as recipient vessels has been shown to reduce overall morbidity, while maintaining the other advantages of the internal mammary vessels-especially reduced operation time and length of hospital stay. For these reasons, the internal mammary perforators could be considered as alternative recipient vessels to replace the thoracodorsal and internal mammary vessels [5-7]. So far, the majority of anatomic and clinical studies of internal mammary vessel perforators have been reported in Western countries, with studies originating from Korea being very rare. Therefore, in this study, we have reported clinical cases of free flap breast reconstruction using internal mammary vessel perforators after confirming whether the internal mammary vessel perforators are reliable as recipient-site vessels in Korean patients. In addition, we have examined the perforating sites, topographic distribution, and external diameter of the internal mammary vessel perforators in patients who have undergone radical mastectomy.
METHODS
Twelve radical mastectomy patients were enrolled between December 2008 and April 2009 among whom reliable internal mammary vessel perforators could be identified preoperatively using a Doppler probe. Almost all of the breast tissue was excised by the oncologic surgeon during radical mastectomy, and the pectoralis major muscle was exposed. Subfascial dissection was executed under 3.5× loupe magnification, and the first to third intercostal spaces were exposed to identify the internal mammary vessel perforators. Fine dissection was performed to obtain enough pedicle length for microscopic anastomosis. The internal mammary artery and vein showed good blood flow after topical papaverine or lidocaine topical application and maximum dilation with microdilators. The external diameter of the perforator vessels was measured with a vessel-measuring gauge from a mechanical coupling device and a paper ruler. The distance from the sternal midline to the location of the internal mammary vessel perforators was also measured (Fig. 1).
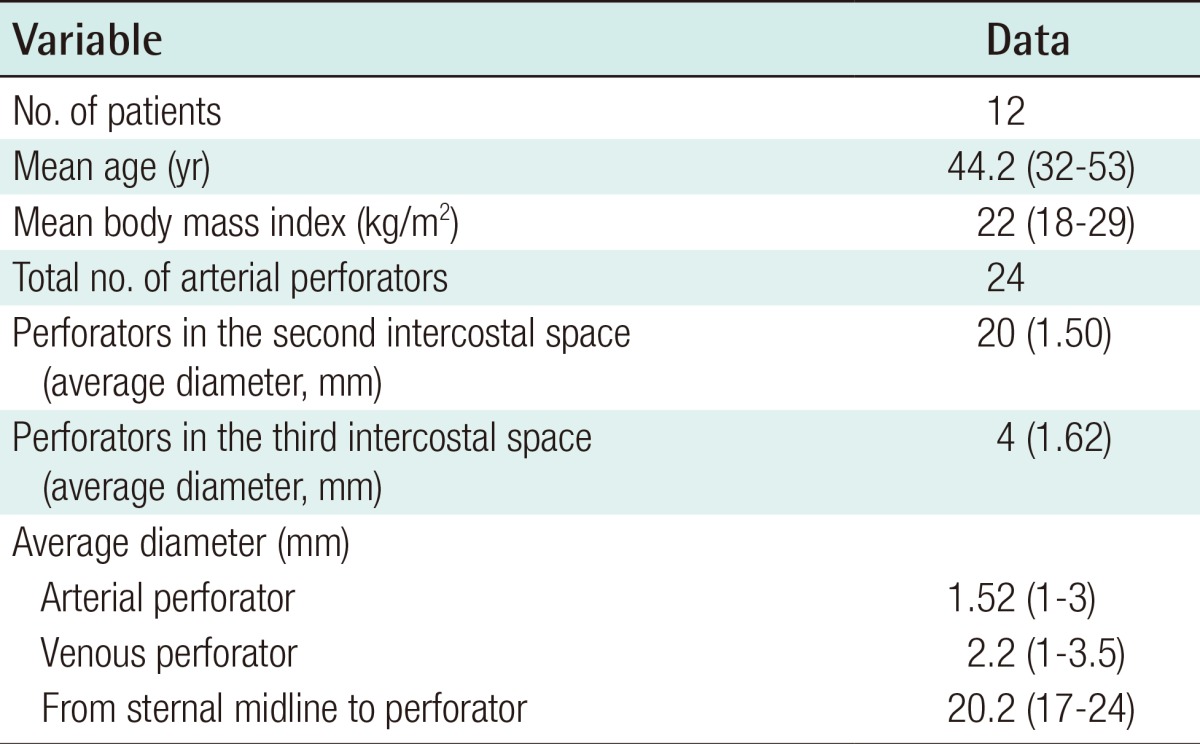
Intraoperative images of internal mammary perforator
(A, B) The intercostal perforating branches from the internal mammary vessels. The perforator was located in the second intercostal space. The arterial and venous diameters were 1.5 mm and 2.0 mm, respectively. (C, D) The external diameter of the perforator vessels was measured with a vessel-measuring gauge from a mechanical coupling device and a paper ruler. The distance from the sternal midline to the location of the internal mammary vessel perforators was also measured.
Breast reconstruction was also performed in 2 patients who had undergone radical mastectomy, for which the free transverse rectus abdominis muscle (TRAM) flap was transferred using internal mammary vessel perforators as recipient vessels on the basis of the formerly identified vessel locations.
RESULTS
Internal mammary vessel perforators were identified at the lateral border of the sternum in all 12 patients (Fig. 1). The average external diameter of the internal mammary vessel perforators was 1.52 mm with a range of 1 to 3 mm, whereas the average external diameter of the venous perforators was 2.2 mm with a range of 1 to 3.5 mm. Considering the intercostal spaces from which these vessels arose, the average external diameter of the arterial perforator at the second intercostal space was larger (1.7 mm) than that in other intercostal spaces. The venous perforator was also largest in the second intercostal space with an average external diameter of 2.5 mm. The average distance from the midline of the sternum to the perforators was 20.2 mm. Twenty perforating vessels, a relatively large number, were observed at the second intercostal space, compared to only 4 perforating vessels observed at the third intercostal space (Table 1).
Breast reconstruction using the free TRAM flap with the internal mammary perforators as recipient vessels was performed in 2 patients who had undergone radical mastectomy, the results of which are shown in Figs. 2, 3.
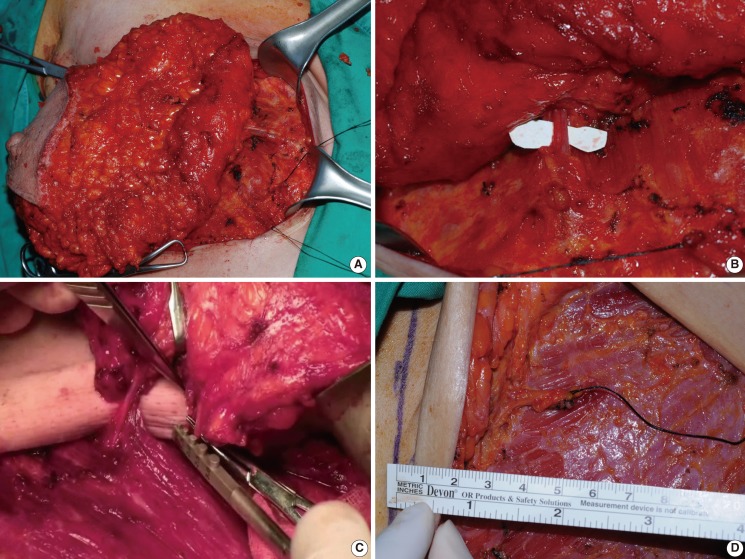
A 40-year-old female with bilateral breast cancer
(A) Preoperative view. (B) Six months after immediate breast reconstruction with a free transverse rectus abdominis muscle flap after modified radical mastectomy. The recipient perforator used was located in the second intercostal space. The arterial and venous diameters were 2.0 mm and 2.0 mm, respectively.
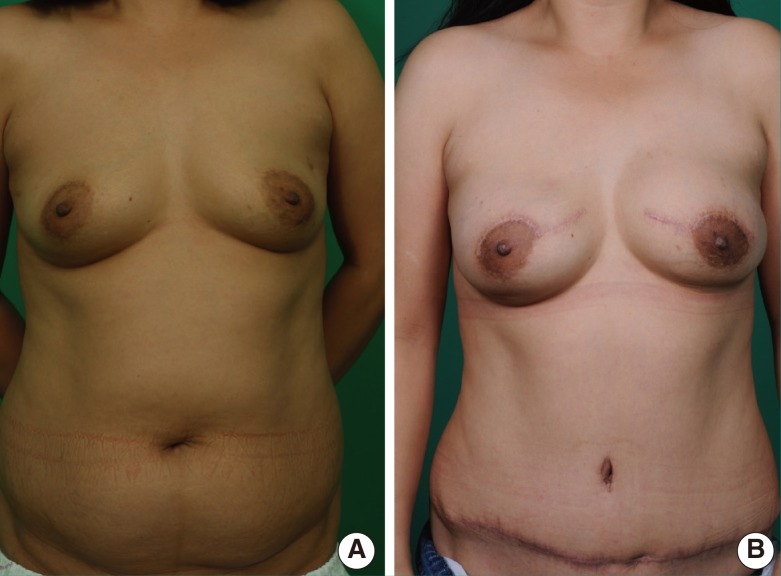
A 38-year-old female with cancer of the right breast
(A) Preoperative view. (B) Immediate breast reconstruction with a free transverse rectus abdominis muscle flap after modified radical mastectomy, postoperative 10 months. The used recipient perforator was located in the second intercostal space. The arterial and venous diameters were 1.5 mm and 2.0 mm, respectively.
DISCUSSION
Selecting proper recipient vessels is very important in breast reconstruction, especially when using perforating vessels. In immediate reconstruction, it is helpful to use the thoracodorsal vessels, which get exposed during the axillary lymph node dissection phase of mastectomy, as recipient vessels. However, in delayed reconstruction, the operative procedure tends to take more time because the fibrosis from the previous mastectomy and radiation therapy surrounding the recipient vessels makes dissection very difficult. Microvascular anastomosis also becomes more difficult due to the reduced diameter of the vessels [2,3].
In such cases, the internal mammary vessels have been frequently used as an alternative to the thoracodorsal vessels. Due to their location, the internal mammary vessels are usually preserved and untouched during mastectomy and postoperative radiation therapy, with no resulting fibrosis or injury, making dissection and microanastomosis easier to perform [6]. In addition, due to their medial origin, using these as recipient vessels allows more volume in the medial area of the breast with better aesthetic outcomes [3,4]. On the other hand, dissecting the internal mammary vessels usually requires partial excision of the costal cartilage, which can lead to morbidities such as chest wall contour defects, pneumothorax, and postoperative pain. Using the internal mammary vessels as a recipient also precludes their usage as donor vessels in future coronary bypass graft surgery [6,8]. Moreover, lymphedema and paresthesia can occur after axillary dissection.
Considering the advantages and disadvantages of the thoracodorsal and internal mammary vessels discussed above, internal mammary vessel perforators may be used as an alternative recipient. Internal mammary perforators branch off the internal mammary artery at the laterodorsal border of the sternum, pass through the intercostal space, pierce the pectoralis major muscle at its medial border, and finally penetrate the overlying fascia [8]. The operating time is reduced since the recipient vessels can be dissected without excision of the pectoralis major muscle or costal cartilage, and a better aesthetic outcome can be expected with augmentation of the medial breast volume since these vessels arise medially to the breast. Since these perforators are located more superficially than the internal mammary vessels, they are less affected by respiratory and cardiac chest wall motion with less vibration, making their microanastomosis easier to perform. Furthermore, the internal mammary vessels are preserved and axillary dissection is avoided, thereby overcoming the disadvantages of both the internal mammary and thoracodorsal vessels [9]. Many studies published in Western countries have discussed the internal mammary vessel perforators, and many clinical cases have been reported [5-7,10]. However, not many anatomic and clinical studies regarding the use of the internal mammary vessel perforators as recipient vessels in Korean patients have been published, with clinical studies being even more uncommon than cadaveric anatomic studies [7].
Surgeons usually hesitate to select internal mammary vessel perforators as recipient vessels because their anatomical locations are deemed less reliable, they have smaller diameters, and their walls are thinner and weaker compared to the internal mammary arteries [9]. However, Hamdi et al. [10] reported the first successful free flap transfer to the internal mammary vessel perforators in 1999. Park et al. [7] performed 5 breast reconstructions with preserved internal mammary vessel perforators as the recipient, and sufficient blood ejection from the severed internal mammary artery vessel perforators was noted in all cases. Haywood et al. [5] found perforators with an external diameter over 1.5 mm and good blood flow in 39% of patients after mastectomy, and achieved good reconstructive results when limiting the use of these perforators as recipient vessels for these selective patients. Munhoz et al. [6] dissected 16 fixed female cadavers and simultaneously performed 40 breast reconstructions using perforators as recipient vessels in a Western population. According to this study, internal mammary vessel perforators were found in only 22 out of 32 parasternal region cadaver dissections, whereas they were found in 72.5% of patients undergoing breast reconstruction. Amid such diverse opinions and controversy concerning the probability of internal mammary vessel perforator existence, we found perforators in all 24 parasternal regions of our 12 patients.
Internal mammary vessel perforators usually consist of one perforating artery and one vein, and the perforating vessels with the largest diameter were located at the second intercostal space [11]. Park et al. [7] reported that a single perforating artery and vein could usually be found at each intercostal space, and that the largest perforating vessels were most frequently observed at the second intercostal space. However, Park et al. [7] also reported that no perforators were found in 22 cadaveric dissections of the second intercostal space, where the largest diameter vessels were usually found. This means that there is a slim but definite chance that perforator vessels would not be found intra-operatively in the second intercostal space, and that preoperative Doppler sonography or multidetector computed tomography angiography studies are necessary for perforator mapping. Park et al. [7] also noted that dissection of other intercostal spaces is necessary to determine the presence of perforators in the absence of such vessels in the second intercostal space when performing breast reconstruction after mastectomy. In accordance with these thoughts, our study found that 16.7% of perforators were located at the third intercostal space.
The diameter of the recipient vessel is one of the major factors in determining the success of free flap reconstruction. When using perforators, which by nature have small diameters, this is even more critical. The diameters of perforating vessels in cadaveric anatomic studies are different from those reported in clinical studies because of postmortem vascular contraction and the absence of pulsation. Munhoz et al. [6] reported that the average arterial perforator diameter was 0.85 mm in 16 fixed cadavers in an anatomic study of a Western population. In 2005, Rosson et al. [12] reported the average diameter of the arterial and venous perforators to be 1.14 mm in 10 fresh cadaveric dissections, also of a Western population. When only the largest perforators were evaluated, the arterial perforators averaged 1.74 mm, and the veins averaged 1.78 mm in diameter. In 2009, Lim et al. [13] reported that the mean external diameter of the arterial perforators was 1.32 mm and the mean external diameter of the venous perforators was 1.48 mm in 11 fixed cadavers. In 2003, Park et al. [7] reported that the average external diameters of arterial and venous perforators in 11 cadaver dissections were 1.32 mm and 1.48 mm, respectively. The largest arterial and venous perforators averaged 1.72 mm and 2.5 mm, respectively. Park et al. [7] also reported that the average external diameters of the arterial and venous perforators in 5 clinical reconstruction cases were 1.32 mm and 1.4 mm, and the largest arterial and venous perforators averaged 2.5 mm and 2.2 mm, respectively.
In our study, the average external diameters of the arterial and venous perforators were 1.52 mm and 2.2 mm, and the largest arterial and venous perforators averaged 3.0 mm and 3.5 mm, respectively. The diameters measured in this study were not smaller than those of other clinical and cadaveric studies. Although technically demanding, successful anastomosis can be achieved by traditional microsurgical techniques in vessels larger than 0.5 mm in diameter. Therefore, the size of the internal mammary vessel perforators is sufficient for them to be considered good candidates for recipient vessels.
Notes
No potential conflict of interest relevant to this article was reported.