Robotic Microsurgery Optimization
Article information
Abstract
The increased application of the da Vinci robotic platform (Intuitive Surgical Inc.) for microsurgery has led to the development of new adjunctive surgical instrumentation. In microsurgery, the robotic platform can provide high definition 12×-15× digital magnification, broader range of motion, fine instrument handling with decreased tremor, reduced surgeon fatigue, and improved surgical productivity. This paper presents novel adjunctive tools that provide enhanced optical magnification, micro-Doppler sensing of vessels down to a 1-mm size, vein mapping capabilities, hydro-dissection, micro-ablation technology (with minimal thermal spread-CO2 laser technology), and confocal microscopy to provide imaging at a cellular level. Microsurgical outcomes from the use of these tools in the management of patients with infertility and chronic groin and testicular pain are reviewed. All these instruments have been adapted for the robotic console and enhance the robot-assisted microsurgery experience. As the popularity of robot-assisted microsurgery grows, so will its breadth of instrumentation.
INTRODUCTION
From the early beginnings of microsurgery, development of instrumentation to assist the microsurgeon in complex microsurgical procedures has been ongoing. The applicability of the robotic platform for microsurgery is improving due to the development of new microsurgery-specific robotic instrumentation. This article will focus on currently available tools and their optimization for robot-assisted microsurgery.
ROBOTIC PLATFORM
Since the first robot-assisted surgery in 1997 by Jacques Himpens and Guy Cardiere in Belgium, the technology has evolved and changed the landscape of modern day surgery. In microsurgery, the 4-arm da Vinci robotic platform (Intuitive Surgical Inc., Sunnyvale, CA, USA) comes with the ability to provide high definition visual 12×-15× magnification, broader range of motion, fine instrument handling with decreased tremor, reduced surgeon fatigue, and improved surgical productivity [1]. As the popularity of robotic assisted microsurgery grows, so will the development of adjunctive instrumentation.
ENDOWRIST ROBOTIC INSTRUMENT
The robotic instruments currently being utilized were initially designed for microsurgical applications in cardiac surgery. The most commonly used instruments in our microsurgical practice are: Black Diamond Micro Forceps, bipolar microforceps, Potts scissors, and monopolar curved scissors (Intuitive Surgical Inc.) (Fig. 1).
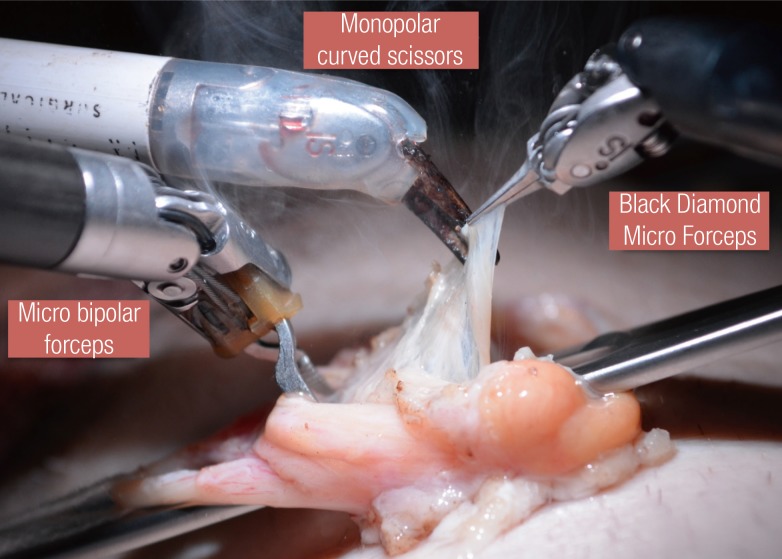
Instrumentation for microsurgical cases
EndoWrist (Intuitive Surgical Inc.) instrumentation for robotic microsurgical cases. The bipolar forceps can be used for cautery, retraction, or suturing. The Black Diamond Micro Forceps can be used for fine dissection, retraction, and suturing.
The instruments are passed through 8-mm trocars, even though the procedure is performed outside the body, to allow the instruments to function with stability. It is important to advance the instruments 4 to 5 cm beyond the tip of the trocar to optimize the range of motion. The Black Diamond Micro Forceps are used for retraction, suturing, micro-dissection, and holding adjunctive microsurgical instruments discussed later in this paper. The bipolar microforceps assist with fine cauterization, suturing, and holding adjunctive microsurgical instruments discussed later in this paper.
TELESCOPIC MAGNIFICATION
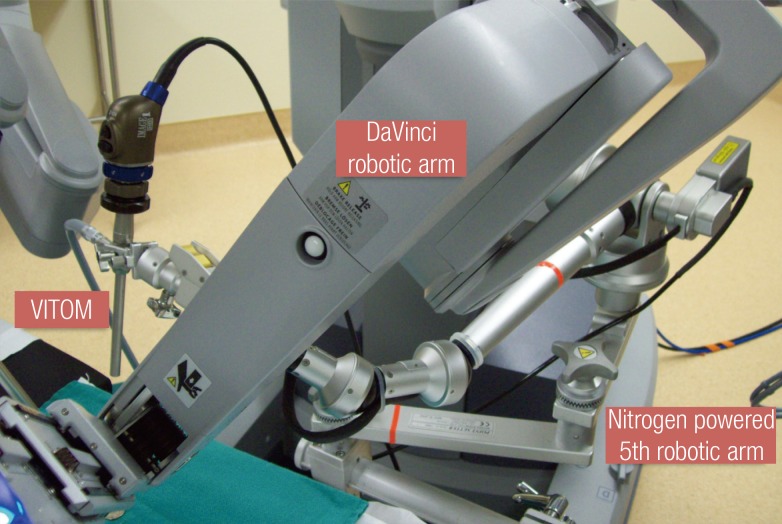
Microsurgical procedures require magnification of small structures. The increased pixilation that comes with digital (12×-15×) magnification on the robotic platform may not provide optimum visualization for microsurgical procedures. The telescope-based high definition video system (VITOM, Karl Storz, Tuttlingen, Germany) offers 16×-18× optical magnification comparable to the operating microscope. Recently, it has even been proposed as a viable alternative for microneurosurgery [2]. For robotic microsurgery, it can provide adjunctive imaging capabilities beyond the 12×-15× digital magnification of the robotic platform. The VITOM can be integrated into the robotic TilePro system (Intuitive Surgical Inc.) that provides the microsurgeon with a cockpit view of all video inputs (Fig. 2). In our practice the VITOM is positioned on an additional fifth robotic (nitrogen powered, Karl Storz) arm (Fig. 3).
Cockpit view of robotic console
(A) View with robotic (10×-15×) magnification. (B) Shows the same image through a higher magnification VITOM lens (16×-20×). (C) Real-time microscopic seen analysis (40×).
We routinely use the VITOM for robot-assisted vasovasostomy (RAVV) and vasoepididymostomy (RAVE). Between July 2007 and March 2013, 147 robot-assisted vasectomy reversals (90 bilateral RAVV, 57 RAVE) were performed using the VITOM. The median patient age was 42 years. 20 of these patients had chronic scrotal pain and the rest wished to regain their fertility. The median time period from vasectomy was 7 years for RAVE and 11 years for RAVE. At a median follow-up of 23 months, men who underwent RAVV and RAVE procedures had a patency rate (>1 million motile sperm/ejaculate) of 97% and 60%, respectively. There was significant pain relief in 88% of the patients who underwent a reversal for post-vasectomy pain. These results are comparable to those of traditional microscopic reversal. The use of robotic assistance with enhanced optical visualization with the VITOM for microsurgical vasectomy reversal may decrease the duration of the operation and significantly improve early semen analysis measures. Further evaluation and longer follow-up is needed to assess its clinical potential and the cost-benefit ratio.
MICRODOPPLER
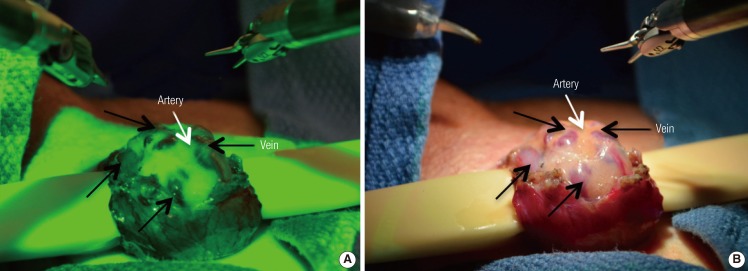
Real time intraoperative Doppler ultrasound use during microsurgical procedures prevents arterial injuries and improves surgical efficiency [3,4]. Standard microscopic Doppler probes (MDP) did not conform to the robotic platform and required the use of a skilled surgical assistant. The flexible drop-in Doppler (Vascular Technology Inc., Nashua, NH, USA) was designed specifically for use with the robotic platform and allows easy manipulation by robotic forceps (Fig. 4). The surgeon can perform real-time Doppler monitoring of the surrounding vasculature and tissue. For microsurgical procedures, the 20-MHz probe can be used for robot-assisted microscopic denervation of the spermatic cord, varicocelectomy, ligation of intracranial aneurysms, and nerve repairs. An alternate 8-MHz flexible drop-in probe is also available for tissues requiring a higher depth of penetration.
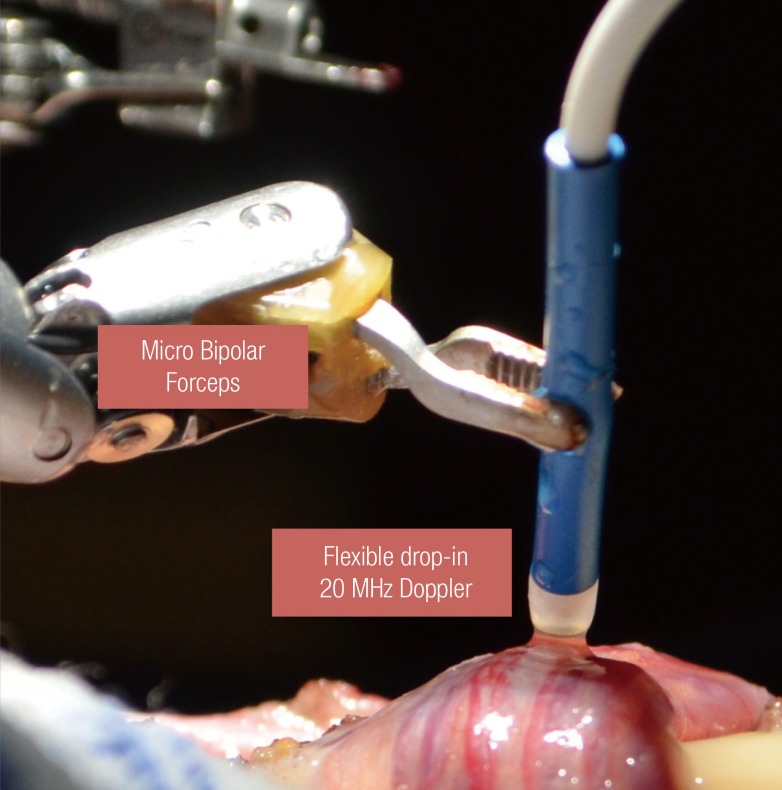
Flexible drop-in micro-Doppler probe
Robot-controlled Doppler probe used to identify testicular arteries and veins during denervation of the spermatic cord.
The flexible drop-in MDP was evaluated in 8 robotic microsurgical cases (3 subinguinal varicocelectomies and 5 spermatic cord denervation procedures) for efficacy in testicular artery localization and ease of robotic grasper maneuverability. It was effective in identifying all of the testicular arteries within the spermatic cord in all cases. Due to the compact size of the MDP, maneuverability using the robotic grasper was significantly improved over the standard handheld Doppler probe. MDP allowed for a full range of motion of the robotic arms allowing the surgeon to easily scan vessels from a wide range of angles without the need for a skilled surgical assistant.
MICRO-ULTRASOUND
A robotically controlled ultrasound transducer (Aloka Inc., Wallingford, CT, USA) has recently been developed that allows surgeons to obtain full ultrasound imaging with real-time flow mapping [5]. Using the robotic graspers to manipulate this probe provides the surgeon with a full range of motion, articulation, and precision when scanning delicate organs (Fig. 5). The use of this probe also negates the need for a skilled surgical assistant. The probe is currently being modified for robot-assisted microsurgery.
HYDRO-JET DISSECTION
Hydro-jet dissection (HD) utilizes a thin, high-pressure stream of water for fine tissue dissection while preserving vascular integrity (Fig. 6). HD can be applied manually or with a pressure-adjustable device such as the ERBEJET2 hydrodissector (ERBE Inc., Tuebingen, Germany). The technology has been successfully applied during open and laparoscopic partial nephrectomy, retroperitoneal lymphadenectomy, and liver resection. Recently, it has been used for fine microsurgical tissue dissection in neurosurgical [6] and urologic procedures. Varying pressure settings can selectively destroy nerve fibers while preserving vascular tissue [7].
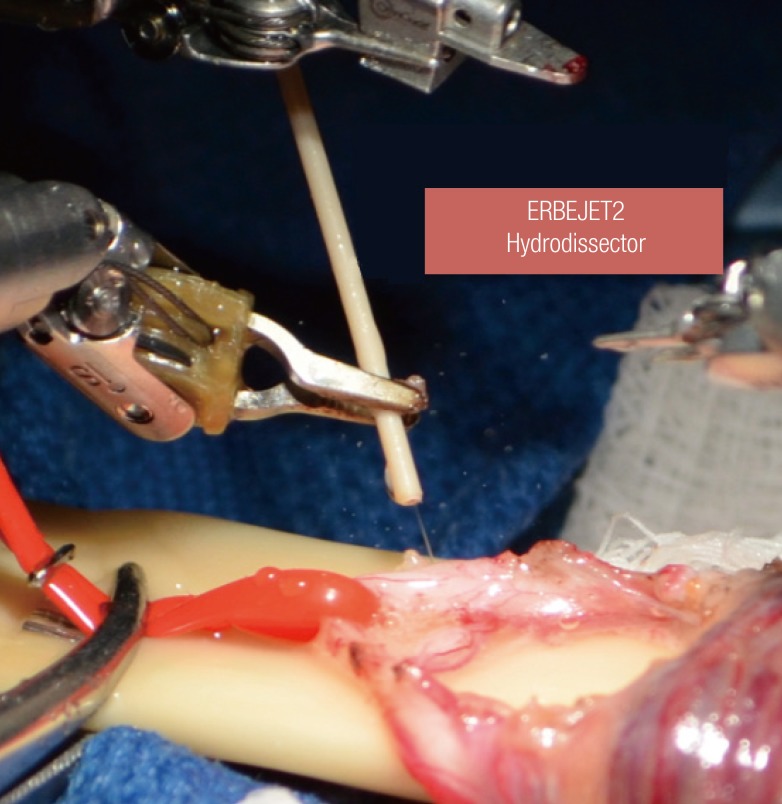
ERBEJET2 hydrodissector for dissection of residual nerves
Hydrodissection of residual perivasal nerves during denervation of the spermatic cord.
There are three primary locations of nerves within the spermatic cord: cremasteric muscle fibers, perivasal tissue and sheath, and posterior peri-arterial/lipomatous tissue [8]. Failures in microsurgical denervation of the spermatic cord (MDSC) can be due to residual small-diameter nerve fibers (≤1 mm) in these locations. A prospective randomized controlled trial was performed on 22 adult rats undergoing bilateral MDSC. HD was performed on one randomized side of the rat cord using 87 psi. The contralateral cord was used as a control (no HD). Cross sections of both cords were examined by a pathologist (blinded). In this study, the HD cords had a lower median residual nerve count compared to the non-HD side, from 8 to 5 nerves (P=0.007). These findings show that HD can successfully ablate small-diameter nerve fibers. This could potentially then translate into improved outcomes after MDSC.
CO2 LASER DISSECTION
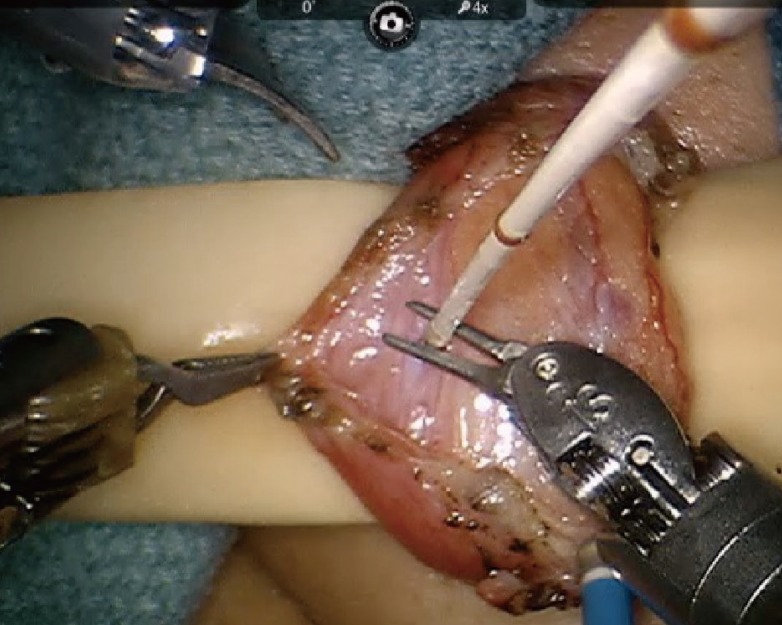
There are many options for ligation or dissection in microsurgery. Monopolar or bipolar cautery continue to be the gold standards even though they can cause significant collateral tissue damage. The CO2 laser is now gaining popularity, given its ability to provide effective ligation and coagulation with less collateral thermal damage [9]. The laser is rapidly absorbed by the water in tissues, thus ensuring minimal thermal damage and spread. This property makes the CO2 laser well suited for dissection and ligation in microsurgery. A bulky delivery system and inability to resect in the microsurgeon's line of dissection previously limited its use in microsurgery. With the advent of the photonic band gap fiber assembly, a flexible fiber-optic CO2 delivery system was developed. The fiber is now integrated into a robotic hand piece (Fig. 7) that is manipulated with robotic arms (FlexGuide ULTRA and Beam-Path Robotic Fiber, OmniGuide, Cambridge, MA, USA).
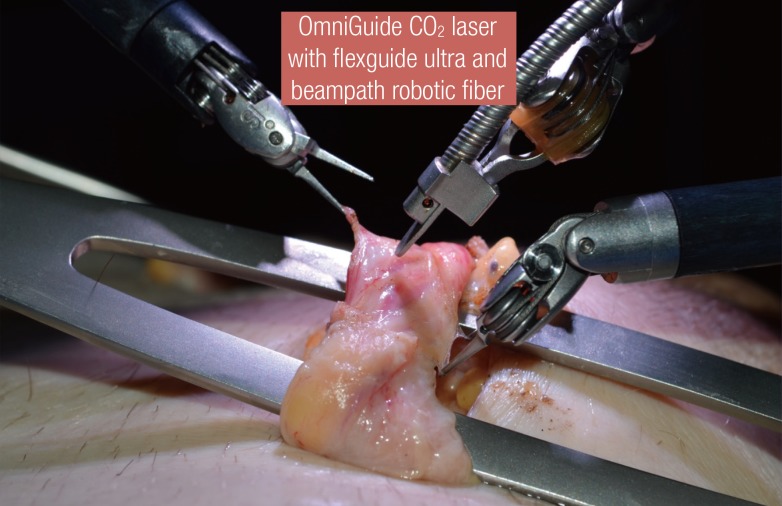
CO2 laser dissection of the spermatic cord
Fine dissection of the spermatic cord using an OmniGuide CO2 laser with robot-specific beampath fiber.
The fiber has been used for robotic-assisted myomectomy [10] for years and recently has been used for microsurgical procedures in otolaryngology [11]. The fiber-optic CO2 fiber was recently compared with the standard cautery for cadaveric vas deferens. Robotic MDSC with the CO2 laser was performed on one spermatic cord side and standard monopolar electrocautery was used on the contralateral side (control). Nine cross sections of the spermatic cord from each side were evaluated by a pathologist (blinded to technique and procedure). On microscopic pathology evaluation, the mean peripheral thermal damage in the spermatic cord was 0.17 mm (range, 0.15-0.25 mm) on the side performed with CO2 laser ablation, significantly less compared to the contralateral monopolar cautery side with 0.72 mm (range, 0.60-0.75 mm) (P<0.001). There was no vascular or vasal trauma evident in either technique. The fiber-optic CO2 laser is a promising alternative to monopolar or bipolar cautery for microsurgical dissection, where it is important to maintain the integrity of the surrounding structures. Studies on the use of this laser for microdissection of the spermatic cord during denervation (for patients with chronic testicular or groin pain) are underway and appear promising.
VEIN VIEWER
The VeinViewer (Christie Digital Systems, Cypress, CA, USA) projects near-infrared light that is absorbed by the blood but reflected by surrounding tissue [12]. This information is captured, processed, and projected on the patient's body with a digital image of the patient's blood pattern. The technology is used for assistance with IV placement or dialysis access. The VeinViewer led to significantly greater likelihood of line placement in infants [13]. This same technology has recently been adopted for microsurgical varicocelectomy for the identification of small diameter veins. The viewer can be positioned over the spermatic cord and veins can be identified in real time (Fig. 8). The technology can be integrated with MDP to enhance the identification of key vascular structures.
CONFOCAL MICROSCOPY
The future of microsurgery appears to be imaging at the cellular level. Confocal microscopy with Cellvizio (Mauna Kea Technologies, Paris, France) allows in vivo histology at a subcellular resolution of tissue inside the body. The small probes are compatible with all flexible video endoscopes and therefore small enough for manipulation by robotic instruments. The probe can be placed directly on the tissue of interest without risk of collateral damage (Fig. 9). Confocal microscopy requires contrast agents for fluorescence imaging such as fluorescein and acriflavine. In a recent study on the enteric nervous system, acriflavine was highly absorbed by neuronal cells, which allowed the clear identification of the enteric nervous system [14]. In the future, confocal microscopy could assist in identification of small residual nerve fibers within the spermatic cord.
CONCLUSIONS
As more microsurgeons venture into robot-assisted microsurgery, the demand for enhanced instrumentation will increase. This will likely drive the industry to develop better tools for microsurgical procedures.
Notes
No potential conflict of interest relevant to this article was reported.