Venous malformations of the head and neck: A retrospective review of 82 cases
Article information
Abstract
Background
Venous malformations (VMs) are a common type of vascular malformation. However, their causes and management remain unclear, and few studies specific to VMs of the head and neck have been reported. This study describes our experiences with VMs of the head and neck.
Methods
This retrospective study included 82 patients who underwent treatment for head and neck VMs, among 222 who visited our vascular anomalies center. Medical records between 2003 and 2016 were reviewed to identify common features in the diagnosis and treatment. The diagnosis of suspected head and neck VMs was based on the results of imaging studies or biopsies, and the VMs were analyzed based on magnetic resonance imaging, computed tomography, and Doppler sonography findings.
Results
VMs were slightly more common in female patients (59.8%), and 45.1% of patients developed initial symptoms at the age of 10 or younger. Lesions were slightly more common on the right side (47.3%). The main sites involved were the cheek (27.7%) and lip area (25.5%). The muscle layer was commonly involved, in 98.7% of cases. Small lesions less than 5 cm in diameter accounted for 60.8% of cases, and well-defined types were slightly more prevalent at 55.4%. Improvement was observed in 77.1% of treated patients.
Conclusions
Early and accurate diagnosis and appropriate treatment according to individual symptoms are important for successful treatment of VMs. If treatment is delayed, the lesions can worsen, or recurrence becomes more likely. Therefore, VMs require a multidisciplinary approach for early and accurate diagnosis.
INTRODUCTION
Vascular anomalies (VAs) were called indiscriminately by various names, and in 1982, Mulliken and Glowacki [1] developed a biological classification system and divided them into hemangiomas and vascular malformations. However, VAs are not differentiated from vascular malformations, and are often misnamed as vascular tumors, which can cause confusion during diagnosis and treatment.
Vascular tumors are neoplastic masses with vascular endothelial hyperplasia, clinically not visible at birth, and involve gradual involution with a clearly proliferative phase after birth. Unlike tumors, venous malformations (VMs) refer to malformations of vascular development that may not be clinically detectable, but are usually apparent in the newborn, with lesions that gradually increase in size. These anomalies are composed of tortuous vascular channels of varying size and shape, lined by a continuous endothelium and surrounded by an abnormal complement of mural cells [2]. Localized errors of angiogenic development are known to cause VMs, but the mechanism is not clearly known. Most VMs are sporadic (94%), although inherited forms exist, of which the most common familial form is glomuvenous malformations (5%) [3]. The incidence rate of VMs is 1 to 2 per 10,000 people, and they can occur anywhere in the body, including the visceral organs, but approximately 40% are known to occur in the head and neck [4].
A systematic study of VMs was carried out in 1982 by Mulliken and Glowacki [1], who proposed a consistent naming system by introducing a biological classification system based on the histopathologic characteristics of endothelial cells. In 1996, the International Society for the Study of Vascular Anomalies (ISSVA) published a classification of VAs according to endothelial cell characteristics, flow characteristics, and clinical behavior. The ISSVA then updated the classification of VAs in 2014. According to the ISSVA, VAs are divided into vascular tumors and vascular malformations. Vascular tumors are divided into three subcategories of neoplastic cellular proliferation and hyperplasia: benign, locally aggressive/borderline, and malignant. In contrast, vascular malformations occur as focal defects of vascular morphogenesis and are classified into four subcategories: simple, combined, those of major named vessels, and those associated with other anomalies [5,6]. Although there have been numerous studies on VMs to date, reports on the clinical features of VMs in Asia are particularly lacking. In this study, we share our experiences of treatment by analyzing the clinical features, distribution and extent, diagnostic process, treatment methods, and treatment outcomes of 82 patients in Korea who were treated for head and neck VMs at our vascular anomalies center (VAC) during a period of 14 years.
METHODS
A retrospective study was conducted of 82 patients who received treatment for head and neck VMs among the 222 VM patients who visited our VAC from 2003 to 2016. Among the four types of VMs according to the ISSVA classification (2014), only subjects with simple VMs were included. Most VAs other than VMs could be differentiated using magnetic resonance imaging (MRI) and Doppler sonography (D-USG); in cases that could not be differentiated using these modalities, biopsy was performed and subjects with types associated with combined or other anomalies on immunohistochemistry were excluded. This study was conducted with the written informed consent of the subjects and was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital prior to conducting the research (IRB No. 2018-10-010).
Based on the medical chart review of each patient, age at diagnosis, sex, treatment methods, and treatment results were confirmed. Except for intracranial VMs, all patients had VMs located in regions extending from the scalp to the neck; these regions were classified as left, center, or right and as upper, middle, or lower based on the glabella and the vermilion border of the upper lip. The location of the VMs was also divided into nine anatomical regions (scalp, forehead, periorbital area, nose, cheek, lip, intraoral area, chin, and neck), and for lesions that spanned two or more regions, each location was counted.
The definitive diagnosis of the patient was mainly made through his or her specific medical history and imaging tests, such as D-USG, computed tomography (CT), and MRI, and in cases of surgical treatment, the biopsy results were confirmed. In most patients, D-USG and MRI were performed on the same day during their first visit. Eight patients were excluded from the analysis of depth and total extent because they underwent only D-USG, which cannot measure these parameters, without MRI or CT. All patients confirmed to have an asymptomatic VM refused further diagnosis or treatment, such patients were then excluded from the study. According to the classification by Goyal et al. [7], treatment outcomes were evaluated by dividing the VMs into four grades based on the size and margin: grade 1, well-defined ≤5 cm; grade 2A, well-defined >5 cm; grade 2B, ill-defined ≤5 cm; and grade 3, ill-defined >5 cm.
According to each patient’s condition, sclerotherapy, surgical resection, and pulsed dye laser or neodymium-doped yttrium aluminium garnet (Nd:YAG) laser (Cynergy, Cynosure Inc., Westford, MA, USA) management were selectively or concurrently used as treatment at the VAC through collaboration with the appropriate department. Laser therapy was excluded from the results because there was no case in which laser therapy alone was performed. The decision of the treatment method(s) was made based on a comprehensive evaluation of the patient’s age, symptoms, location of the lesion, grade, complications, and cosmetic factors. After treatment, outcomes were evaluated by the VAs team in three stages, including an assessment of symptoms, a physical examination, and an imaging test, to confirm the size of the lesion, and the following outcome assessments were assigned: poor, little or no improvement; good, significant decrease in size and symptoms; and excellent, radiological obliteration [7]. The imaging modality used to evaluate the treatment outcomes was most often D-USG, although MRI was re-performed if aggravated or necessary.
RESULTS
Clinical features
A total of 222 patients with pure VMs who were diagnosed at our VAC over the past 14 years were included in the study. Among them, 82 patients had head and neck VMs (36.9%). Of these subjects, 33 (40.2%) and 49 (59.8%) were men and women, respectively, showing a slight predominance of the condition among women. Thirty-seven of the 82 patients (45.1%) had received their first diagnosis before turning 10 years old. The average age of diagnosis was 18.3 years (range, 1 month to 70 years), and it was 19.3 years in women and 16.6 years in men. Sixty-three patients had first sought treatment at our institution, while 19 came from the other local medical centers. The average follow-up period was 4.4 years, including 12 patients who were lost to follow-up.
Type, distribution, and location
The type, distribution, and location of the lesions were evaluated through physical examination and imaging tests in patients in whom a VM was suspected. With the exclusion of the eight patients who underwent only D-USG, the multifocal type was found in five patients (6.8%) and the unifocal type in 69 patients (93.2%) on MRI or CT scans. The lesion size was less than 5 cm in 45 patients (60.8%) and more than 5 cm in 29 patients (39.2%). The margin was well-defined in 41 patients (55.4%) and ill-defined in 33 patients (44.6%).
Among the 74 patients with information from MRI or CT scans, VMs occurred more often on the right side (35 patients, 47.3%) than on the left side (32 patients, 43.2%), and were most common in the lower region (30 patients, 36.6%), followed by the middle-lower region (24 patients, 29.3%), and the middle region (14 patients, 17.1%). Altogether, the right lower region was the most common incidence region (Fig. 1). When the location of the head and neck VMs was divided into nine regions, the most common region was the cheeks (39 patients), followed by the lips (36 patients) and the chin (20 patients), and 38 patients had two or more affected regions (Fig. 2).
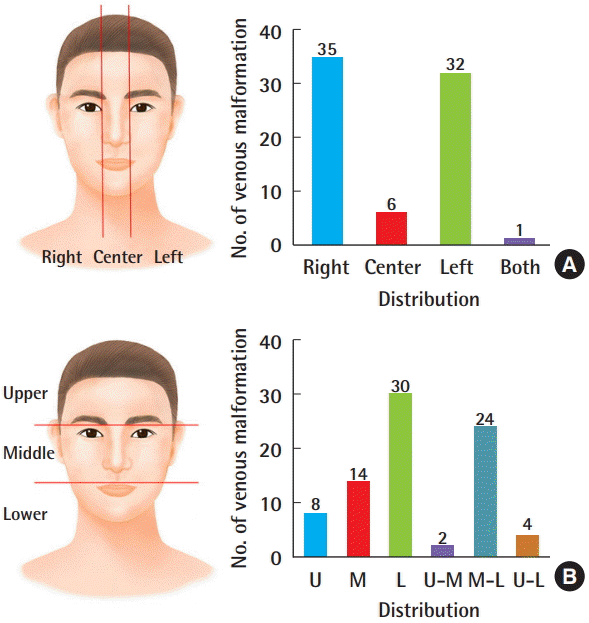
Distribution of venous malformations (n=74)
(A) The numbers on the left and right were similar and (B) the lower region was predominant. U, upper region; M, middle region; L, lower region.
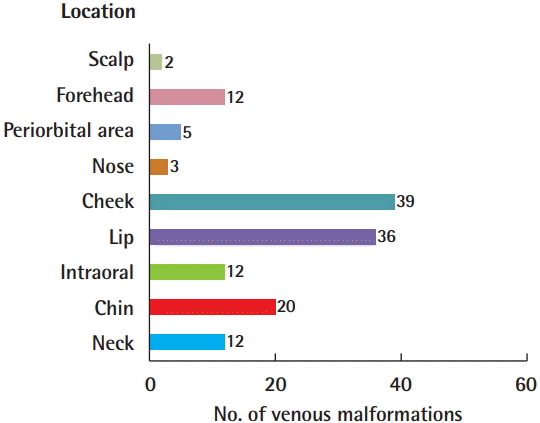
Location of venous malformations
Venous malformation was most commonly present in the cheek (39 patients) and lip (36 patients). If a malformation was present in two or more regions, each location was counted (n=141).
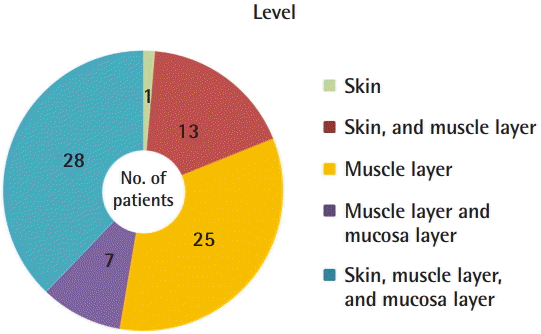
With the exclusion of the eight patients in whom this information could not be identified, the VMs in the 74 remaining patients were classified based on the depth and level of invasion, as follows: the skin (including subcutaneous tissue), the muscle layer, and the mucosa layer. The muscle layer was invaded in 98.7% of patients, but in one patient (1.3%), the VM was confined to the skin, including the subcutaneous tissue. The most common region of invasion was the skin (including subcutaneous tissue) along with the muscle and mucosa in 28 patients (37.8%), followed by the muscle layer only in 25 patients (33.8%), the skin (including subcutaneous tissue) and the muscle layer in 13 patients (17.6%), and the muscle and mucosa layers only in seven patients (9.5%) (Fig. 3).
Diagnosis
Fig. 4 shows the algorithm used for the diagnosis and treatment of patients suspected to have a vascular mass in the head and neck. Each patient’s diagnosis was finalized in collaboration with the various departments within the VAC and was confirmed through imaging tests or biopsy, mostly after a physical examination and history-taking in patients suspected to have a vascular malformation.
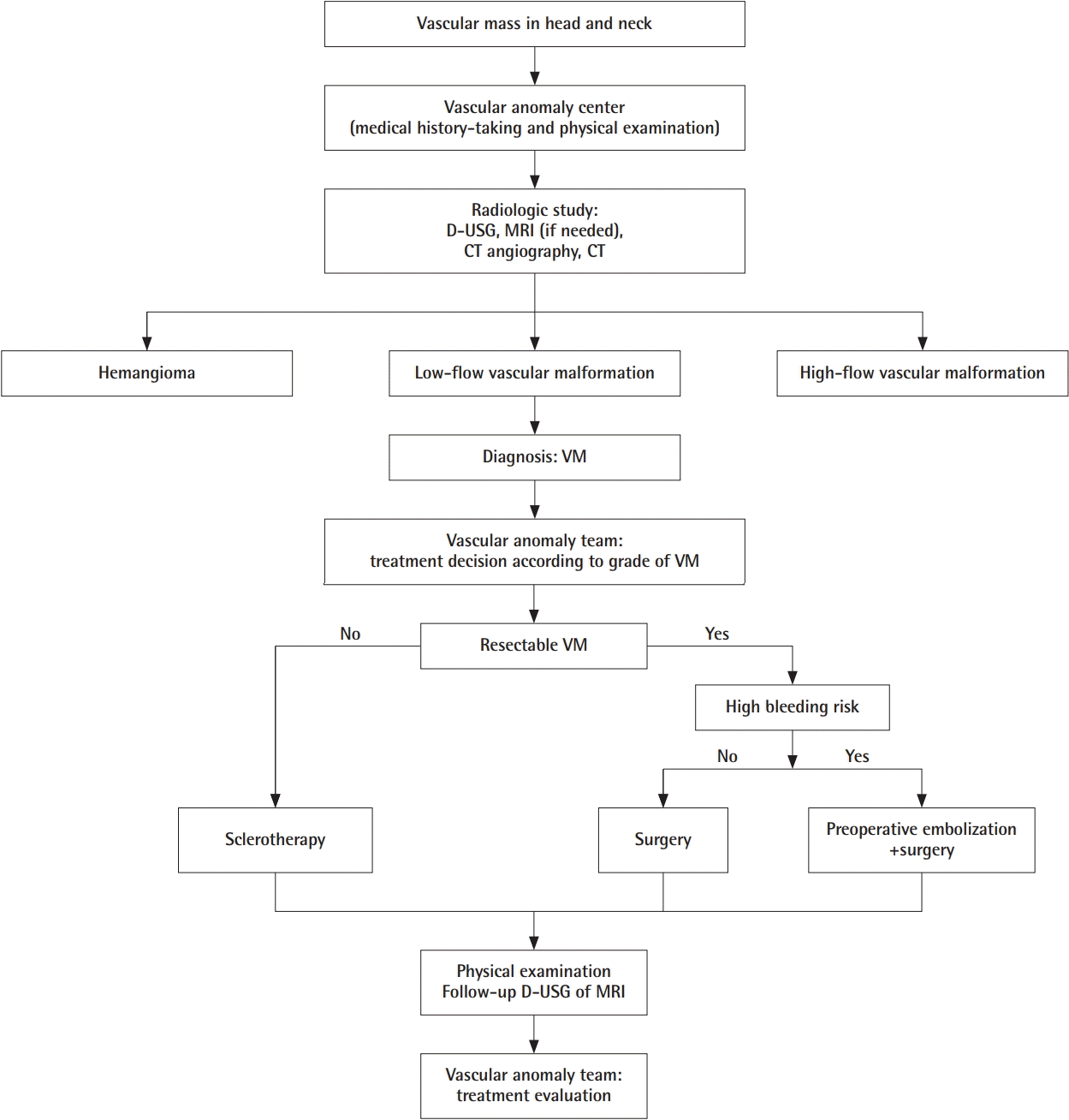
Algorithm for management of venous malformation
The algorithm used at Kyungpook National University for the diagnosis and treatment of patients with suspected vascular anomalies. D-USG, Doppler sonography; MRI, magnetic resonance imaging; CT, computed tomography; VM, venous malformation.
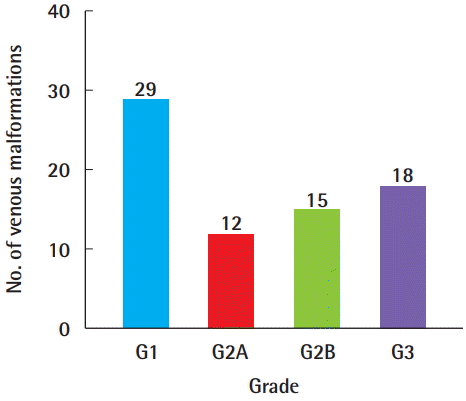
Imaging tests were performed with CT, MRI, and D-USG. Of the 82 subjects, 68 (82.9%), 12 (16.2%), and 60 (81.1%) underwent MRI, CT, and D-USG examinations, respectively; two patients (2.7%) underwent imaging after a punch biopsy at another local medical center, while two patients (2.7%) underwent surgical resection without imaging for simultaneous diagnosis and treatment. In 58 patients (78.4%), two or more tests were performed, including an imaging test and biopsy, 14 patients (18.9%) were treated after a single test, and seven patients each underwent MRI and D-USG. In the first diagnostic workup, D-USG was performed in 36 patients (48.6%) and MRI in 34 patients (45.9%), and both MRI and D-USG were performed in 31 patients (41.9%) around the same time within a week. Due to the burden of radiation, CT was performed only when the size of the lesion and its relationships with adjacent structures needed to be clarified. When dividing the grade by the size and margin after an MRI or CT scan, a grade 1 VM was found in 29 patients (39.2%), a grade 2A VM in 12 patients (16.2%), a grade 2B VM in 15 patients (20.3%), and a grade 3 VM in 18 patients (24.3%) (Fig. 5).
Treatment
Sclerotherapy was performed 2.54 times on average as the main treatment method, and it was the only treatment in 40 of the 82 patients (48.8%), except for two patients under observation. With the inclusion of patients who also underwent surgery during the treatment period, sclerotherapy was performed in a total of 66 patients (78.6%). Sclerotherapy was performed prior to debulking surgery for totally unresectable lesions, and was also performed in cases of significant functional impairment or cosmetically severe deformities, or for uncontrolled symptoms such as severe pain or swelling and bleeding. Sclerotherapy was also performed when there was concern about massive intraoperative bleeding, or when VMs involved major organs, with a risk of impaired vision, hearing, or eating.
For sclerotherapy, the VM was directly punctured under fluoroscopy, and contrast was used to identify the VM territory before injection; depending on the sclerosant, a maximum of 0.15 mL/kg of alcohol (range, 0.5–7.5 mL), from 0.5 mL of 0.5% sodium tetradecyl sulfate (STS) foam to 6.0 mL of 3.0% STS foam (range, 0.5%–3.0%; 0.5–6.0 mL), or 0.1–2.3 U/kg of bleomycin foam (1 mg/1 mL=1 U; range, 1–35 U) was used. Considering age, sex, body weight, the lesion’s location, the depth of invasion, and complications, STS, bleomycin, and alcohol were selected or used in parallel.
Surgery alone was performed in 14 patients (17.1%), while in 26 patients (31.7%), a combination of surgery and sclerotherapy was performed. Surgery was either complete resection or debulking. Complete resection was performed for small to moderate-sized well-defined VMs that did not invade vital structures. Debulking surgery was performed for unresectable lesions, to provide symptom relief before sclerotherapy, or when bulkiness remained after sclerotherapy. Surgery was performed with due consideration of postoperative complications such as scarring.
Prior to the surgical resection of large VMs, a decision was made whether to prevent intravascular coagulopathy by using low-molecular-weight heparin. Laser therapy was performed in 11 patients after sclerotherapy or surgery.
The treatment outcomes showed improvement in 27 of the 40 patients (67.5%) treated with sclerotherapy alone and in 16 of the 26 patients (61.5%) who underwent sclerotherapy with surgery. Of the total of 66 patients who underwent sclerotherapy with or without surgery, 43 (65.2%) showed improvement, whereas nine (64.3%) of the 14 patients who had surgery only showed improvement (Table 1).
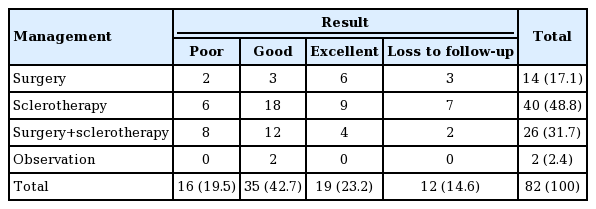
Treatment outcomes of venous malformations in the head and neck area according to treatment methods (n=82)
In terms of management by the grade of the VM, sclerotherapy alone was the most frequent treatment in grade 1, grade 2A, and grade 3 VMs, whereas in grade 2B VMs, sclerotherapy along with surgery was the most common. Grade 1 VMs were most frequently treated with sclerotherapy (15 patients, 50.0%), followed by surgery with sclerotherapy (9 patients, 30.0%). Grade 2A VMs were most frequently treated with sclerotherapy (6 patients, 54.5%), followed by surgery (3 patients, 27.3%). Grade 2B VMs were most frequently treated by surgery with sclerotherapy (6 patients, 40.0%), followed by sclerotherapy (4 patients, 26.7%) and surgery (4 patients, 26.7%). In grade 3 VMs, sclerotherapy was the most common treatment (9 patients, 50.0%), followed by surgery with sclerotherapy (7 patients, 38.9%).
Good results were observed most often in all grades (grade 1, 36.7%; grade 2A, 54.5%; grade 2B, 40.0%; grade 3, 50.0%). In grade 1 VMs, the next most common results were excellent in nine patients (30.0%) and poor in five patients (16.7%). In grade 2A VMs, excellent results were obtained in three patients (27.3%) and a poor result in one patient (9.1%), and in grade 2B VMs (15 patients), excellent results occurred in three patients (20.0%) and a poor result in one patient (6.7%). In grade 3 VMs (18 patients), the results were poor in seven patients (38.9%) and excellent in two patients (11.1%) (Table 2).
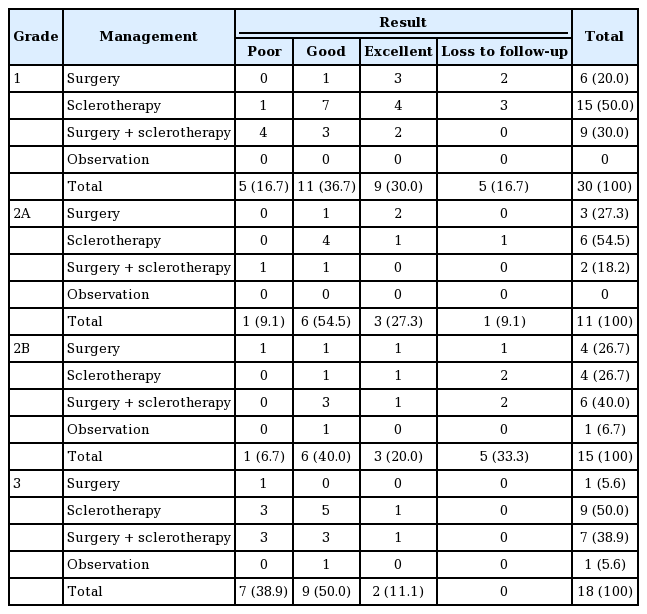
Treatment methods and outcomes of venous malformation in the head and neck area according to stage (n=74)
Post-treatment complications occurred in three of the 82 patients (3.7%), and two discontinued treatment due to delirium or skin blanching during sclerotherapy. The other patient developed skin necrosis after sclerotherapy. All three patients with complications improved with conservative management.
Cases
Case 1
A 29-year-old female patient visited our hospital with pain on mastication and a palpable mass in the left cheek that had developed 1 year previously. After taking a medical history and a physical examination, the patient was transferred to the VAC with a suspected VM (Fig. 6A). First, a detailed medical history and physical examinations were conducted by team conference, and VM was diagnosed by MRI and D-USG (Fig. 6B and C). The mass was a grade 1 well-defined lesion located anterior to the masseter muscle. The mass was small with a clear margin, and an interdisciplinary team performed surgery, determining that it would be possible to achieve complete resection without leaving an external scar via an intraoral approach (Fig. 6D). The patient showed satisfactory results at 4 months postoperatively.
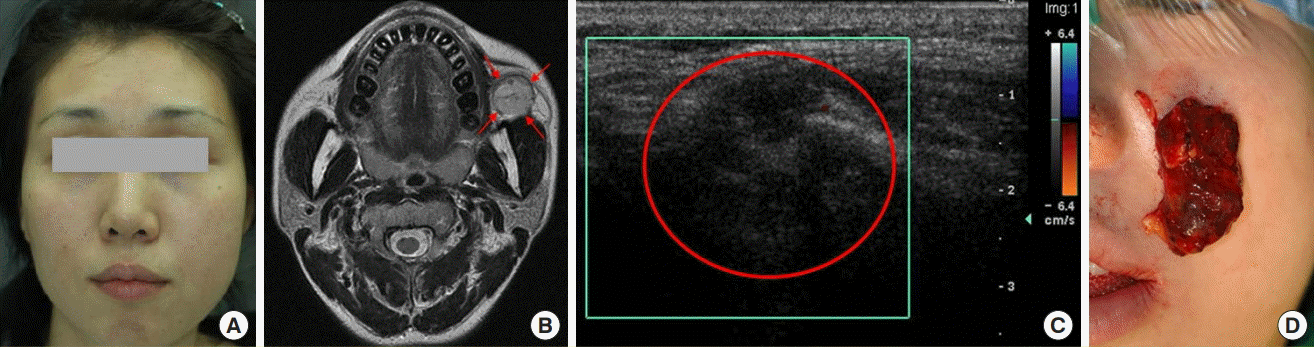
A case of VM with surgical treatment
(A) Preoperative photograph. (B) Preoperative MRI, T2 phase, axial view, with the red arrows indicating the high signal intensity of the VM lesion. (C) Preoperative D-USG image, where the red circle indicates the hypodensity of the VM lesion. (D) Intraoperative photograph, surgery was performed with an intraoral approach without leaving an external scar. VM, venous malformation; MRI, magnetic resonance imaging; D-USG, Doppler sonography
Case 2
An 8-year-old male patient underwent radiofrequency ablation at another hospital 3 months previously for a palpable mass and facial deformity in the right temporal area, but the lesion did not improve and the patient visited the VAC (Fig. 7A). First, a detailed medical history and physical examination were conducted via a team conference, and VM was confirmed by MRI and D-USG (Fig. 7B and C). The mass was a grade 2A well-defined lesion. Since the margin was clear, the interdisciplinary team considered surgery; however, due to the anatomical location, the risk of facial deformity due to facial nerve injury was high, and the team decided to perform sclerotherapy (Fig. 7D). At a 1-year follow-up after sclerotherapy, D-USG and MRI showed no remaining VM outside of the VM nest (Fig. 7E). An interdisciplinary team conference determined that the mass had been completely obliterated, and the patient was scheduled for continuous follow-up.
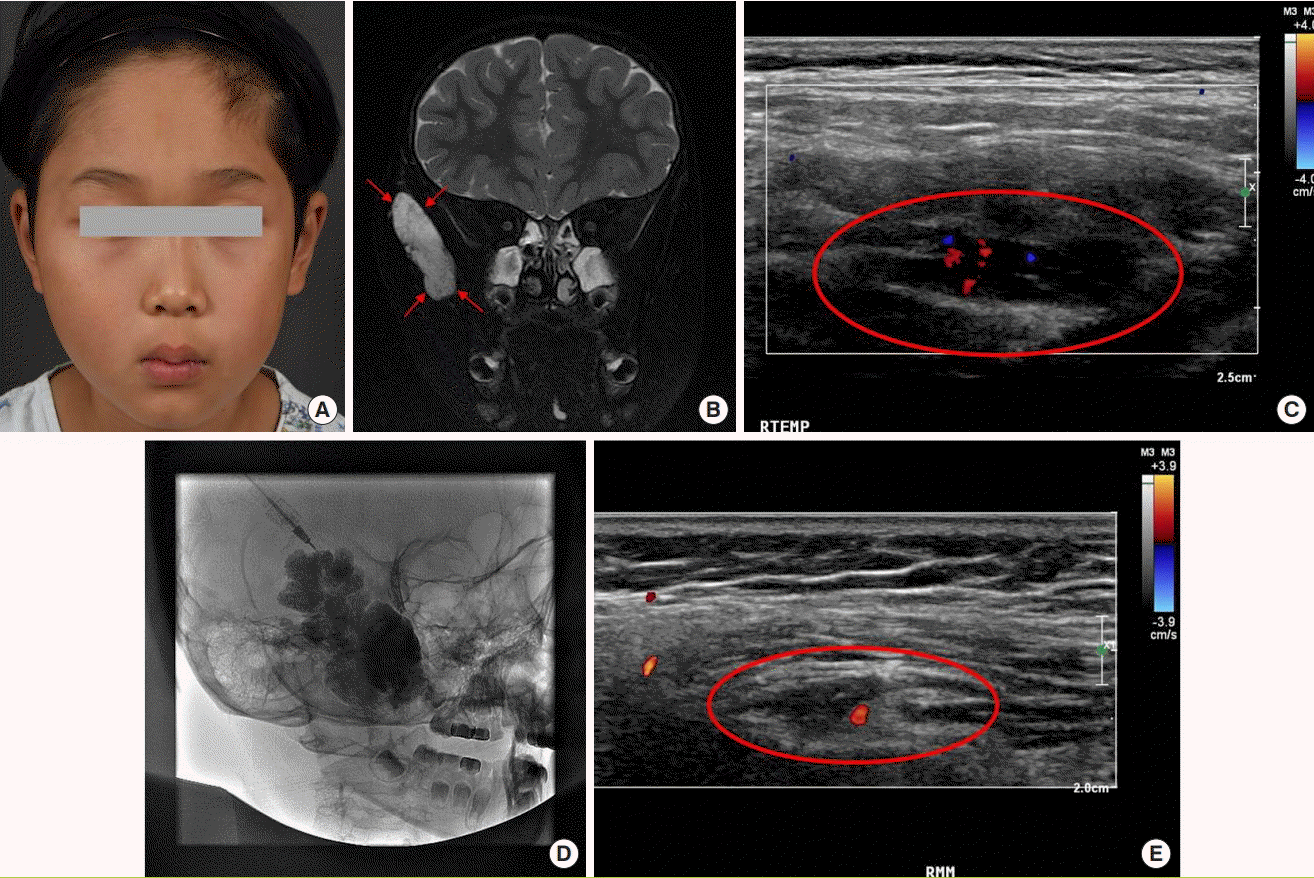
A case of VM with sclerotherapy
(A) Clinical photograph before sclerotherapy. (B) Pretreatment MRI, T2 phase, coronal view, with the red arrows indicating the high signal intensity of the VM lesion. (C) Pretreatment D-USG image, where the red circle indicates the hypodensity of the VM lesion. (D) Intra-sclerotherapy photograph, contrast medium injection into the malformation through an angiocatheter before the injection of sclerosant. Bleomycin was used as the sclerosant. (E) D-USG at 1-year post-sclerotherapy, the VM had improved. The red circle indicating no residual lesion. VM, venous malformation; MRI, magnetic resonance imaging; D-USG, Doppler sonography.
Case 3
A 3-year-old male patient underwent sclerotherapy using polidocanol at another hospital at the age of 3 months, due to a left cheek mass that was suspected to be a VM. The mass did not improve, and the patient visited the VAC at our hospital (Fig. 8A). In the medical history taken by the VAs team, the patient showed speech problems and pain when eating. Physical examination showed a huge mass in the left cheek accompanied by tenderness. The vascular team performed MRI and D-USG (Fig. 8B and C), surgery was scheduled in a conference based on the image tests and symptoms, and the patient showed relief of symptoms caused by the huge mass (Fig. 8D). In D-USG at 4 months postoperatively, no residual lesion was observed. However, in subsequent follow-up at 6 months postoperatively, the VM had recurred, and additional sclerotherapy was performed using alcohol, STS foam, and bleomycin to remove the remaining mass (Fig. 8E). Follow-up imaging after sclerotherapy showed partial improvement (Fig. 8F), and based on an evaluation of treatment outcomes at an interdisciplinary team conference, a management plan was established.
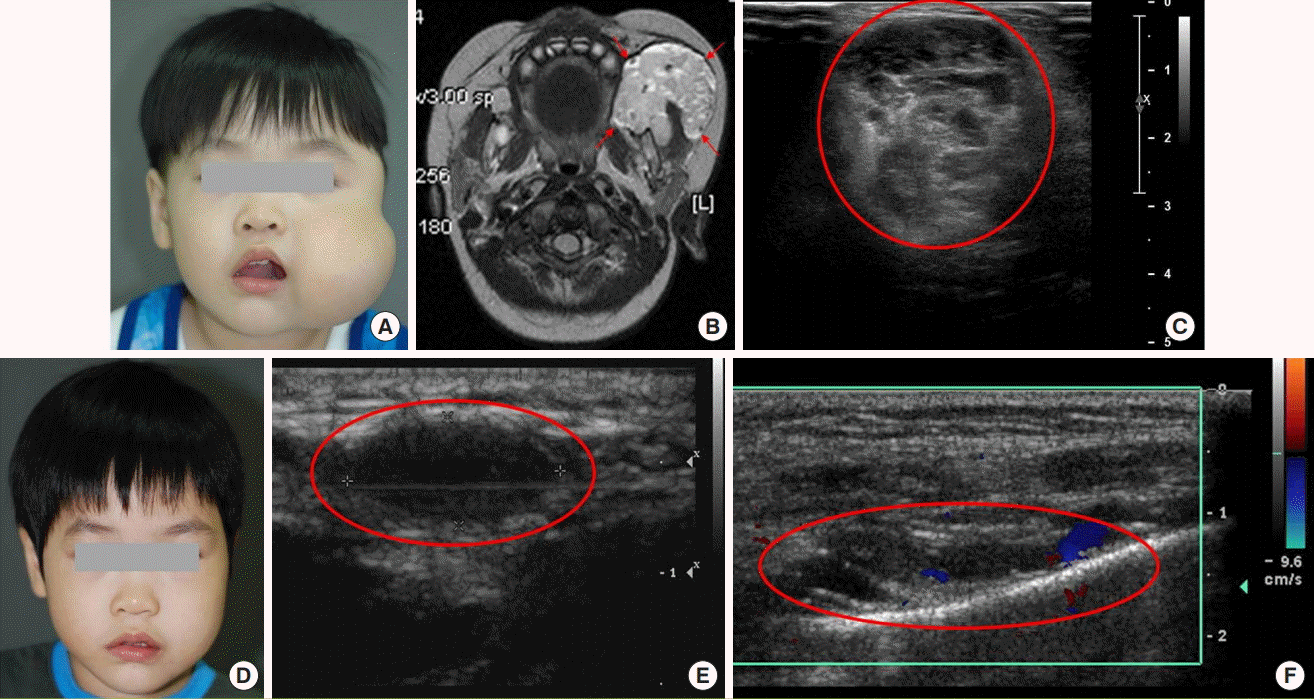
A case of VM with combinational treatment
(A) Clinical photograph before treatment. The patient had a huge mass on the left cheek. (B) Pre-treatment MRI, T2 phase, axial view, with the red arrows indicating the high signal intensity of the VM lesion. (C) Pre-treatment D-USG image, where the red circle indicates the VM lesion. (D) Postoperative photograph, showing that the symmetry of both cheeks had improved. Complete resection was performed with careful attention to avoid facial nerve injury. (E) D-USG at 6 months after resection, the red circle indicates recurred lesion of VM. Additional sclerotherapy was performed using alcohol, sodium tetradecyl sulfate foam, and bleomycin. (F) D-USG image at 5 months post-sclerotherapy. The red circle indicates partial improvement in the lesion. VM, venous malformation; MRI, magnetic resonance imaging; D-USG, Doppler sonography.
DISCUSSION
A specific medical history and physical examination are important for the diagnosis of VMs. Unlike involuted hemangioma, VMs do not regress until adulthood and are usually non-pulsating soft masses with black and blue skin discoloration. A key clinical feature of VMs is their sensitivity to pressure (compression) from sources such as the finger, which causes them to change size, and they may also enlarge due to gravity, the Valsalva maneuver, or straining. VMs can be painful masses and can occur anywhere in the body, including in the skin, subcutaneous tissue, muscle, visceral organs, joints, and the central nervous system, and VMs can also occur at multiple sites. In 61.9% of patients with VMs, pain was present when they woke up in the morning, and the pain or size increased in 73.9% of patients due to hormonal changes (puberty, menstruation, and pregnancy), although pain was not caused by applying compression [8-10].
On MRI, VMs are identified as high-signal-intensity lesions on T2 enhancement and low-signal-intensity lesions on T1 enhancement. MRI can identify the depth of invasion, distribution, location of the lesion, and aspect, and are classified using a grade that takes into account their size and margin, thereby contributing to the decision of the treatment plan [7]. CT can be used to observe phleboliths, which are characteristic of VMs. D-USG is a cost-effective primary diagnostic tool that can observe low-flow, low-shunt vascular dynamics, and VMs appear as hypoechoic masses on D-USG. However, D-USG is operator-dependent, which is a disadvantage because it cannot precisely observe deeply located lesions.
VMs are classified as sporadic or inherited. Sporadic VMs are mostly unifocal, whereas some inherited forms often manifest as multifocal. Sporadic, unifocal types account for 93% of cases, whereas sporadic, multifocal types account for only 1%. Inherited forms are often multifocal, with 1% of cases being cutaneomucosal VMs and 5% glomuvenous malformations [11].
Traditional treatment modalities for VMs include compression garments, laser therapy, sclerotherapy, and surgery [12]. As difficulties are encountered in curing all VMs in the same way, the treatment plan should be determined considering the extent and clinical symptoms of the VM and the patient’s age.
Sclerotherapy is a nonsurgical form of management, and endovascular therapy is preferred as a treatment method. Injection sclerotherapy using STS foam for children with oropharyngeal VMs has been reported to be a safe and effective treatment option, and percutaneous treatment with bleomycin foam has also been reported to be effective. However, after sclerotherapy, skin blistering, skin necrosis, ulceration, scarring, and airway obstruction due to swelling may occur [13-16].
The efficacy of surgical management has been demonstrated by the remission or improvement of disease after excision or debulking surgery in patients with VMs who did not respond to conservative therapy or sclerotherapy. After patients with VMs who had symptoms of pain, contour deformity, and skin discoloration underwent surgery, their appearance, function, and overall quality of life improved [17,18].
Laser treatment has been reported to remove the bluish discoloration and to reduce the size of the lesion after Nd:YAG laser treatment for superficial VMs. Other studies have reported excellent treatment results for pain and bleeding control, as well as reductions in lesion size after long-pulse Nd:YAG laser therapy for VMs in the lip and oral mucosa [19,20].
Several researchers have studied the methods and effects of VM treatment, but a unified treatment strategy has not been determined. Therefore, the treatment plan should be made after considering all the patients’ symptoms, the location of the lesion, and its depth, size, type, and sequelae. The authors considered sclerotherapy first. Surgery was performed when it was determined that a radical cure was possible after consideration of the extent, site, and complications. Furthermore, if treatment was impossible by sclerotherapy alone, surgical treatment was performed alone or in combination with other treatments.
A multidisciplinary approach is needed for the diagnosis and treatment of VMs because it is easy to misdiagnose or decide on a wrong treatment in the existing single-treatment system. Previous studies have reported a correct diagnosis rate of only 37% by comparing the previous diagnostic history at other medical institutions to that of a medical center specializing in vascular tumors and vascular malformations [21]. According to another study, only 22.4% of the previous diagnoses were accurate among patients who visited the VAC at the University of California, San Francisco (USA) for the first time [22]. Moreover, a multidisciplinary approach involves the participation of specialists from various fields, which has the advantage of giving the patient and medical staff a broader range of treatments to choose from.
With the exclusion of 12 patients (14.6%) who were lost to follow-up, 19.5% showed poor results, 42.7% showed good results, and 23.2% showed excellent results. Considering the high recurrence rate of VMs and the difficulty of radical cure, the therapeutic outcomes of this study were positive. However, the lack of family history and genetic studies means that the relationship of treatment results with a genetic diagnosis could not be determined. Although an accurate diagnosis and proper treatment are considered important for the management of VMs, a standard protocol has not yet been determined for its diagnosis and treatment, and further studies are necessary. If more data on the diagnosis and treatment are accumulated, it will be possible to reach a standard protocol by consensus. However, as of now, our study indicates that a helpful approach to the management of VMs will be treating each case in an appropriate manner through collaboration among specialists within the VAC.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Kyungpook National University Hospital (IRB No. 2018-10- 010) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.