The use of botulinum toxin type A to minimize scarring in cleft lip repair: A literature review
Article information
Introduction
Postsurgical scars can be a major concern for many patients. Young and Hutchison [1] found that patients were usually not satisfied with their surgical scars, with 91% stating that they would value any further improvement in their scars. Cleft lip with or without cleft palate is one of the most common congenital anomalies worldwide [2,3]. Nonsyndromic cleft lip/palate affects approximately 1.5 to 2.5 cases per 1,000 live births, and its epidemiological distribution varies by ethnicity and geographic area [4,5]. In today’s culture, there is a major emphasis on facial appearances, largely driven by advances in social media. Individuals with deviations from what is considered “a normal look” may be socially stigmatized in their communities. Therefore, the final facial scar appearance remains a significant concern for affected individuals and their families. Herein, we review the process of wound healing and summarize the literature evaluating the effects of botulinum toxin type A (BTA) on scar formation in cleft lip repair.
Wound healing
Incisional wounds heal through a dynamic process requiring intricate coordination among multiple cell types and an appropriate extracellular environment [3,6,7]. Any disruption to this microenvironment may impair healing, resulting in pathological scar formation. Therefore, it is of great importance to recognize and appropriately manage both systemic and local factors that may adversely affect incisional wound healing and scar appearance [7]. The healing process is composed of four distinct phases: homeostasis, inflammation, proliferation, and remodeling [3,6]. The remodeling phase begins at the 3rd week of injury and may take up to 2 years to complete. During this phase, disorganized, immature, and relatively weak type 3 collagen is replaced with mature type 1 collagen [3,6]. Fibroblasts mature into myofibroblasts for wound contraction, and proteases degrade existing disordered tissue, resulting in scar reduction and maturation [3,6]. A key factor that may unfavorably impact healing, leading to pathological scars, is underlying tension acting on a healing wound [8,9]. For instance, scars tend to widen when underlying forces that pull the wound edges apart are applied to newly formed type 3 collagen before it fully matures [8,9]. Furthermore, the opposing forces caused by underlying muscular contractions inflict, at the cellular level, subclinical injuries to the healing wound [8,9]. This repetitive micro-trauma drives an escalated inflammatory response, resulting in more fibrosis, which in turn increases the risk of hypertrophic scar formation [9].
Many surgical and postsurgical techniques are commonly used to minimize wound tension, such as tissue undermining, deep sutures, taping, scar massaging, and silicone sheeting [9]. Such techniques, however, decrease underlying wound tension, but do not eliminate it. In case of cleft lip repair, the underlying tension is primarily caused by the orbicularis oris muscle, which is in constant use during daily life for speech, drinking, eating, and making a variety of facial expressions [10]. Current data suggest that BTA injections after incision closure might significantly optimize cosmetic outcomes, thereby obviating the need for scar-enhancing modalities, such as further revision surgery. BTA temporary paralyzes the underlying muscle, eliminating the dynamic muscle tension on the healing wound and minimizing scarring [8]. It selectively binds to the receptor sites on cholinergic nerve terminals, decreasing the presynaptic release of acetylcholine, the principal neurotransmitter at the neuromuscular junction; this, in turn, results in chemical denervation of the muscle and a localized significant reduction in muscle activity [11,12]. The neuromuscular blockade is temporary, usually lasting from 3 to 6 months.
Animal studies have elucidated that the role of BTA in wound healing extends beyond decreasing muscular tension. In addition, BTA diminishes the inflammatory response, as shown by decreased expression of transforming growth factor-β1 and a reduced fibroblastic response without disturbance of epithelial growth; thus, it promotes better healing and an improved clinical scar appearance [13,14]. These data laid the foundation for human use and clinical trials. In 2006, Gassner et al. [15] published the first prospective, blinded, randomized, placebo-controlled study designed to investigate whether BTA-driven chemo-immobilization could improve the aesthetic appearance of forehead scars to a statistically significant extent. Following the study of Gassner et al., other clinical trials investigating surgical facial scar minimization by BTA injections were conducted [16-19].
BTA in cleft lip repair
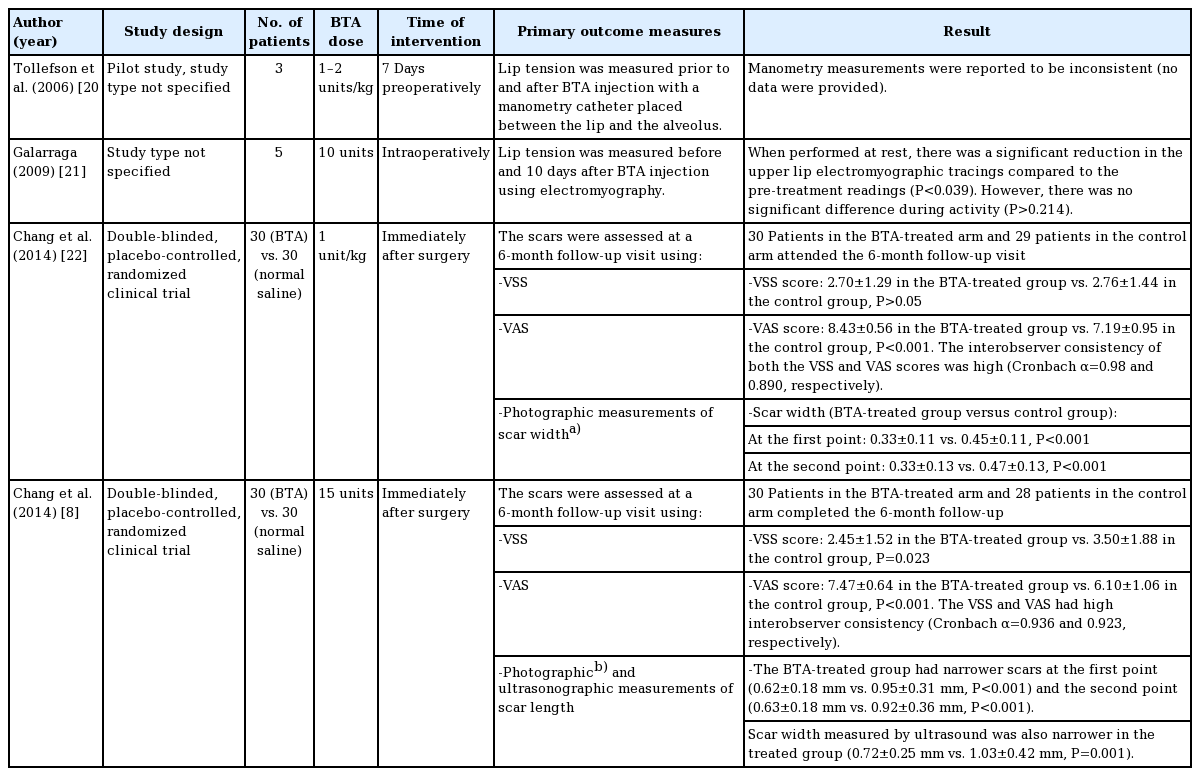
Several studies have investigated the effects of BTA in cleft lip repair (Table 1). Tollefson et al. [20] reported the use of BTA in three children undergoing primary cleft lip repair. Seven days prior to surgery, three infants (aged, 3–6 months) received BTA injections into the orbicularis oris with a total dose of 1–2 units/kg. The authors reported satisfactory results with no toxin-related complications. Although the scars were reported to be satisfactory, the study lacked any subjective or objective scar assessment tools to support such a conclusion. Galarraga [21] showed that intraoperative BTA injections significantly reduced orbicularis oris activity when measured by electromyography. An electromyographic study was conducted in the upper lip of five infants (aged < 6 months) before surgery. The patients were then treated with 10 units of BTA intraoperatively before surgical repair. Ten days postoperatively, repeated electromyograms at rest showed a significant reduction in electromyographic tracings compared with pre-injection tracings (P < 0.039). No significant difference in tracings was noted during upper lip activity (P > 0.214), most likely due to the involvement of other perioral muscles that did not receive a BTA injection.
Chang et al. [22] conducted a prospective double-blinded, placebo-controlled, randomized clinical trial of 60 infants, 59 of whom completed the study, with unilateral cleft lip who were scheduled to have primary surgical repair around the age of 3 months. Half of the patients received BTA injections immediately after surgical repair and the other half (the control group) received normal saline injections. At a 6-month follow-up visit, two plastic surgeons independently assessed the patients using the Vancouver Scar Scale (VSS). The mean VSS scores of the two evaluators were used. The VSS is one of the most frequently used scar assessment tools, and the score of each scar is based on an assessment of its pigmentation, vascularity, pliability, and height [13]. Using Photoshop, scar width was measured by two independent raters at two defined points: “the first point was 1 mm above the white roll and the second point was 1 mm below the C-flap suture line” [22]. Furthermore, five examiners (plastic surgeons and laypersons) independently graded the scars using a Visual Analogue Scale (VAS), ranging from 0 (worst possible scar) to 10 (best possible scar). All evaluators were blinded regarding patients’ specific group allocations. The VSS assessments showed no significant differences between the two groups. The patients in experimental group had significantly narrower scars than those in the control group at both the first (0.33 ± 0.11 vs. 0.45 ± 0.11, respectively; P < 0.001) and the second previously defined anatomical points (0.33 ± 0.13 vs. 0.47 ± 0.13; P < 0.001). In addition, the VAS was significantly better in the BTA-treated group (8.43 ± 0.56 vs. 7.19 ± 0.95; P < 0.001). None of the patients developed any complications such as wound dehiscence, oral incontinence, or feeding difficulty.
Chang et al. [8] reported another prospective double-blind, placebo-controlled, randomized clinical trial of 60 adults who underwent cleft lip scar revision surgery between 2010 and 2012. Patients were randomized to receive either BTA or normal saline injections into the adjacent orbicularis oris muscle following wound closure. A total of 58 patients who completed 6 months of follow-up were assessed using the VSS, VAS, and photographic and ultrasound measurements of scar width. The outcome assessors were blinded to which group the patients belonged. The scars of patients in the experimental group were shown to be aesthetically superior to those patients in the control group as the experimental group showed statistically better outcomes in all scar assessment modalities with high interobserver consistency. No complications were reported. Although there were no BTA-related complications in either study, larger clinical trials with well-defined dosage and long-term follow-up are needed to better examine the safety and clinical effectiveness of BTA in cleft lip repair.
Conclusion
Current clinical studies suggest that BTA injections may prevent cleft lip hypertrophic scarring but are insufficient to provide significant support for its use as the standard of clinical care in cleft lip surgical repair. The sample size was relatively small in most of the studies, and objective assessment tools of scar improvement were not thoroughly used. Trials with good methodological quality and a large sample size are needed to reach firm conclusions in terms of the aesthetic use of BTA for cleft lip scar enhancement. Moreover, no study has yet investigated the cost-effectiveness of this intervention compared with a control group and the potential need for further surgical and nonsurgical scar enhancement techniques. Finally, further investigations of long-term safety are needed.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Alhazmi B, literature review, writing–original draft; Aldekhayel S, writing–critical review & editing. All authors read and approved the final manuscript.