Indocyanine green fluorescence videoangiography for reliable variations of supraclavicular artery flaps
Article information
Abstract
Background
Pedicled flaps are useful for reconstructive surgery. Previously, we often used vascularized supraclavicular flaps, especially for head and neck reconstruction, but then shifted to using thoracic branch of the supraclavicular artery (TBSA) flaps. However, limited research exists on the anatomy of TBSA flaps and on the use of indocyanine green (ICG) fluorescence videoangiography for supraclavicular artery flaps. We utilized ICG fluorescence videoangiography to harvest reliable flaps in reconstructive operations, and describe the results herein.
Methods
Data were retrospectively reviewed from six patients (five men and one woman: average age, 54 years; range, 48–60 years) for whom ICG videoangiography was performed to observe the skin perfusion of a supraclavicular flap after it was raised. Areas where the flap showed good enhancement were considered to be favorable for flap survival. The observation of ICG dye indicated good skin perfusion, which is predictive of flap survival; therefore, we trimmed any areas without dye filling and used the remaining viable part of the flap.
Results
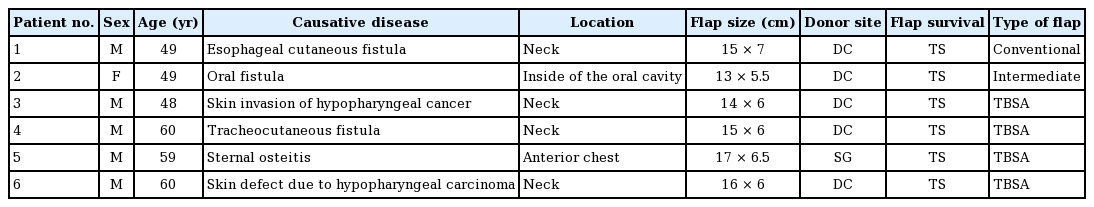
The flaps ranged in size from 13×5.5 cm to 17×6.5 cm. One patient received a conventional supraclavicular flap, four patients received a TBSA flap, and one patient received a flap that was considered to be intermediate between a supraclavicular flap and a TBSA flap. The flaps completely survived in all cases, and no flap necrosis was observed.
Conclusions
The TBSA flap is very useful in reconstructive surgery, and reliable flaps could be obtained by using ICG fluorescence videoangiography intraoperatively.
INTRODUCTION
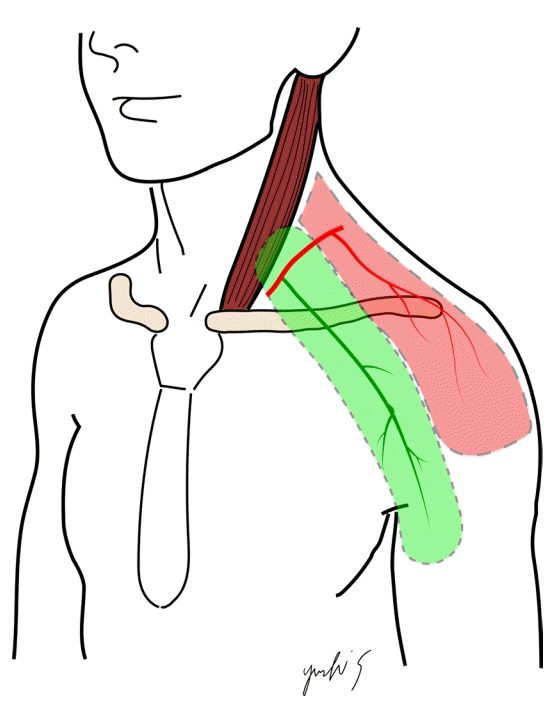
Although free flaps are often used in head and neck reconstruction, they involve technical problems such as vascular anastomosis and invasion due to the extended operative time; therefore, pedicle flaps remain very useful for such procedures. The supraclavicular flap is a highly reliable pedicled flap with stable blood perfusion, and many studies have described its anatomical features [1-3]. Recently, studies of the thoracic branch of the supraclavicular artery (TBSA) flap have reported the possibility of placing the skin paddle in the anterior thorax, as opposed to the conventional supraclavicular artery flap (Fig. 1) [4,5]. Previously, we used supraclavicular flaps in our practice, but have gradually shifted to using TBSA flaps. However, few anatomical studies have been performed of TBSA flaps. In addition, to our knowledge, no previous studies have reported the intraoperative use of indocyanine green (ICG) fluorescence angiography in supraclavicular artery flaps. We have experienced good results using ICG fluorescence videoangiography while harvesting supraclavicular artery flaps and TBSA flaps in reconstructive operations, and we describe the results herein.
METHODS
Data were reviewed from six patients (five men and one woman) with an average age of 54 years (range, 48–60 years) for whom ICG videoangiography was performed during construction of a supraclavicular artery flap at the authors’ institution. All patients who presented with defects requiring flap surgery in whom an operation was performed between May 2017 and August 2018 were included. The underlying diseases of the patients were as follows: postoperative skin defects of an esophageal cutaneous fistula, defects associated with resection of hypopharyngeal cancer combined with skin invasion, a postoperative oral fistula due to maxillary gingival cancer, a postoperative tracheocutaneous fistula due to tongue cancer, sternal osteitis, and skin defects after the resection of hypopharyngeal carcinoma. The relevant data were gathered retrospectively from intraoperative pictures or movies and the medical records of each patient. All patients provided informed consent for the surgical procedures and use of their images, and the procedures were performed according to our institutional standards. Institutional review was not required for this retrospective data review study.
Flap harvesting
Preoperatively, we verified the basal part of the supraclavicular artery using color Doppler vascular ultrasonography and confirmed the presence of blood flow in the flap direction. While harvesting the TBSA flap, we cut the flap toward the deltopectoral fossa, incised it from the distal side of the fascia, and removed it as a fasciocutaneous flap. During these steps, caution was required because the cephalic vein runs through the deltopectoral fossa. After confirming that the pedicle ran along the clavicular bone and blood flowed into the flap, we separated the surrounding tissue. When it was judged that the flap could sufficiently cover the defect, ICG fluorescence videoangiography was performed to confirm the blood perfusion of the flap.
ICG fluorescence videoangiography
The process of ICG fluorescence videography is shown in Supplemental Video 1. First, 25 mg of ICG was dissolved in 10 mL of sterile distilled water, of which 2 mL was injected into the intravenous line. Subsequently, the flap was observed using the PDE-Neo device (Hamamatsu Photonics, Hamamatsu, Japan). By placing thick gauze beneath the flap before the intravenous injection of ICG, it was possible to prevent imprecision caused by the excitation light generated from the intact surrounding skin.
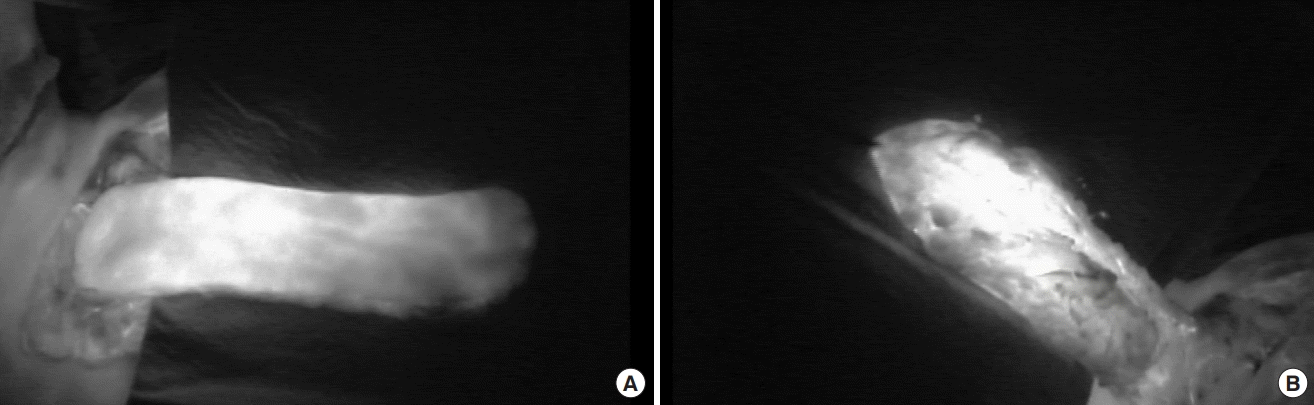
After the intravenous injection of ICG, we recorded a video of the ICG angiography of the harvested flap to confirm that the flap filled with dye quickly. The presence of ICG dye uptake indicated good skin perfusion, which is predictive of flap survival (Fig. 2); we then trimmed any areas without dye filling and used the remaining viable part of the flap.
RESULTS
One patient received a conventional supraclavicular flap, four patients received a TBSA flap, and one patient received a flap that was considered to be intermediate between a supraclavicular flap and a TBSA flap (Table 1). The sizes of the flaps ranged from 13×5.5 cm to 17×6.5 cm. In one patient, it was necessary to harvest a skin graft from the de-epithelialized flap skin for donor site wound closure; in all other patients, the donor site was closed directly. The flaps completely survived in all cases, and no cases of flap necrosis were observed.
A 60-year-old man with type 2 diabetes mellitus had undergone tracheotomy, glossectomy, and laryngectomy for tongue cancer, followed by reconstruction with a free rectus abdominis myocutaneous flap. Postoperatively, the posterior wall of the tracheal pit was fistulized (Fig. 3A), and a huge dead space was identified in the anterior cervical portion; hence, we decided to perform surgery to close the fistula.
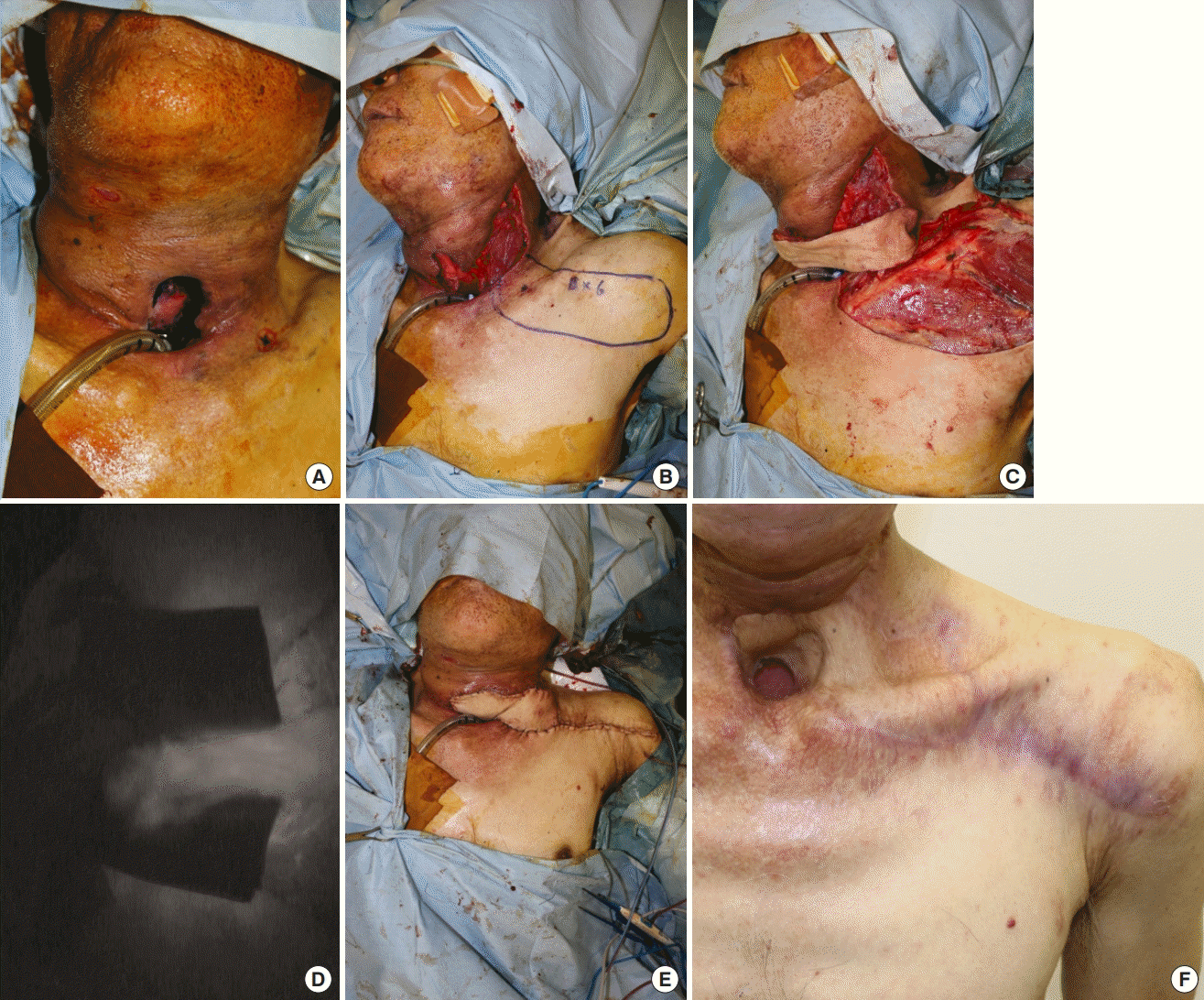
A case of TBSA flap
(A) The posterior wall of the tracheal pit was fistulized. (B) A 15×6-cm TBSA flap was harvested. (C) The flap was rotated and sutured. (D) The flap was fully dyed by indocyanine green, indicating good skin perfusion. (E) After checking that the flap was fully enhanced, we sutured it. (F) The condition of the flap was good at 6 months postoperatively. TBSA, thoracic branch of the supraclavicular artery.
First, an incision was made along the surgical scar from the fistula. Second, we debrided the area of poor granulation tissue. Since the oral cavity and neck were connected, we anchored the skin to the neck in order to block the leakage. Subsequently, a TBSA flap measuring 15×6 cm was harvested (Fig. 3B). The flap pedicle was fashioned from the surrounding tissues, and the flap was rotated 180° and sewn to the posterior wall of the tracheal pit (Fig. 3C). The presence of ICG dye in the flap indicated good skin perfusion (Fig. 3D); therefore, we used the entire flap and sutured it (Fig. 3E).
Postoperatively, there was no recurrence of the fistula around the trachea, and no obvious infection was observed even after the patient started oral intake of food (Fig. 3F).
DISCUSSION
To our knowledge, no studies to date have described the use of ICG fluorescence angiography for harvesting a supraclavicular artery flap. Furthermore, the anatomy of TBSA flaps has not been well researched. Therefore, we used ICG fluorescence videoangiography for harvesting supraclavicular artery flaps and TBSA flaps to ensure reliable results following surgery. Although the small study population is a limitation of this study, all the harvested flaps survived. This result indicates that ICG fluorescence videoangiography could be useful for harvesting these flaps.
The supraclavicular flap is a fasciocutaneous flap designed using a vascularized supraclavicular artery. After the supraclavicular flap was first reported by Lamberty [6], it was used as an island flap by Pallua et al. [1]; subsequently, it was used for burn scar reconstruction of the cervix, and it is currently used for head and neck reconstruction. The advantages of the supraclavicular flap include a shorter operative time than is needed when using a free flap [2]. Additionally, microsurgical techniques can be difficult to perform in hospitals with few microsurgeons; hence, the supraclavicular flap may be the first choice of flap, depending on the hospital setting.
The supraclavicular artery runs from the transverse cervical artery through the thyrocervical trunk, rises to the skin at the posterior triangle of the neck, and runs through the platysma as a perforator artery. According to anatomical studies [7], the conventional supraclavicular flap is also vascularized from choke vessels of an artery of the deltoid muscles; it is reportedly possible to harvest a flap about 30 cm long from the upper arm [5]. However, reconstruction of the head, neck, and anterior thorax is often performed with the patient in the supine position. Particularly when using conventional methods, it may be difficult to undermine the dorsal side.
In our clinical practice, we gradually modified the design of the skin paddle to an anterior supraclavicular flap design and confirmed that there was no problem with skin perfusion by using ICG fluorescence videoangiography. Eventually, we started using the TBSA flap, which is designed along the direction of the deltoid-pectoral fossa. As a result, the surgical approach with the patient in the supine position became very easy to perform. It is reportedly possible to harvest a TBSA flap approximately 35 cm long by extending the harvest by about 5 cm compared with the conventional method [5]. It is also possible to raise a thinner, supple flap, and this type of flap has shown excellent results in facial reconstruction [5]. The rate of total necrosis of the flap associated with using the anterior branch of the supraclavicular artery is less than 5% [4,5], and the TBSA flap is very reliable. Nevertheless, anatomical studies of the TBSA flap are lacking, and according to the available studies, the blood vessel required for this flap exists only in about 60% of the overall population [8]. However, in clinical reports that preceded the use of the TBSA flap, this blood vessel branch was recognized in all individuals [4,5]; thus, further anatomical research is warranted to provide clarification of this issue. We increased the reliability of flap survival by additionally performing ICG fluorescence videoangiography, and no cases of skin necrosis were observed.
With respect to ICG fluorescence videoangiography, the PDE-Neo device used herein emits light at a wavelength of 760 nm, which activates ICG; thus, we clearly identified ICG in the blood, and perfusion of the flap could be readily seen. Flap imaging with ICG videoangiography predicts indicates survival of the flap with high accuracy [9,10], and it is a very useful method for flap harvesting.
In addition, the use of ICG videoangiography preoperatively is also useful for identifying the pedicle of the supraclavicular flap. ICG is not inferior to computed tomography angiography in identifying the pedicle [11], and ICG videoangiography has the advantage of not exposing the patient to radiation. Our experience confirms that ICG angiography is extremely useful preoperatively and intraoperatively for obtaining reliable results when harvesting supraclavicular artery and TBSA flaps. Further, our findings suggest that using ICG angiography intraoperatively could be an effective method of predicting flap survival.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: Suzuki Y, Shimizu Y. Data curation: Suzuki Y, Shimizu Y, Kasai S, Yamazaki S, Takemaru M, Kitamura T, Kawakami S, Tamura T. Formal analysis: Suzuki Y. Methodology: Suzuki Y, Shimizu Y. Project administration: Suzuki Y, Shimizu Y. Visualization: Suzuki Y. Writing - original draft: Suzuki Y. Writing - review & editing: Suzuki Y. Approval of final manuscript: all authors.
Supplementary Material
Supplemental Video 1. This video demonstrates how to perform indocyanine green fluorescence videoangiography. It is a useful technique for harvesting a reliable flap.
Supplemental data can be found at: https://doi.org/10.5999/aps.2018.01536.v001