Incidental finding of subclavian artery occlusion and subsequent hypoplastic internal mammary artery as a candidate recipient vessel in DIEP flap breast reconstruction
Article information
Abstract
We report a case of autologous breast reconstruction in which a thoracodorsal vessel was used as a recipient vessel after a hypoplastic internal mammary vessel was found on preoperative computed tomography (CT) angiography. A 46-year-old woman with no underlying disease was scheduled to undergo skin-sparing mastectomy and breast reconstruction using a deep inferior epigastric artery perforator flap. Preoperative CT angiography showed segmental occlusion of the right subclavian artery with severe atherosclerosis and calcification near the origin of the internal mammary artery, with distal flow maintained by collateral branches. The thoracodorsal artery was selected to be the recipient vessel because CT showed that it was of adequate size and was not affected by atherosclerosis. The patient experienced no postoperative complications, and the flap survived with no vascular complications. The breasts were symmetrical at a 6-month follow-up. This case highlights that preoperative vascular imaging modalities may help surgeons avoid using diseased vessels as recipient vessels in free flap breast reconstructions.
INTRODUCTION
In autologous tissue-based breast reconstruction, the deep inferior epigastric artery perforator (DIEP) flap has become the gold standard technique. According to Lie et al. [1], the failure rate of DIEP flaps is about 0%–3%. A reliable recipient vessel is critical for avoiding flap failure. The internal mammary vessels that originate from the subclavian artery are the most common and reliable recipient vessels in breast reconstruction using a DIEP flap [2]. The antegrade internal mammary artery (IMA) and retrograde IMA are commonly used as recipient vessels [3,4]. In this report, we present a case of incidentally found subclavian artery occlusion and subsequent hypoplastic IMA discovered during a preoperative evaluation for autologous breast reconstruction.
CASE
A 46-year-old woman with a body mass index of 24.34 kg/m2 was scheduled to undergo skin-sparing mastectomy on her right breast and immediate breast reconstruction using a DIEP flap. She was a non-smoker and had no underlying disease such as hypertension, diabetes mellitus, or peripheral arterial occlusive disease (PAD).
On preoperative computed tomography (CT) angiography, segmental occlusion and severe atherosclerosis were identified in the right subclavian artery, with calcification near the origin of the right IMA. The distal flow of the IMA was maintained by collateral branches (Fig. 1). Although the flow was preserved by collateral branches, the right IMA was smaller than the left IMA, suggesting decreased blood flow (Fig. 2). The thoracodorsal vessels were adequate in size and were without atherosclerosis (Fig. 3); therefore, the thoracodorsal artery was chosen to be the recipient vessel.
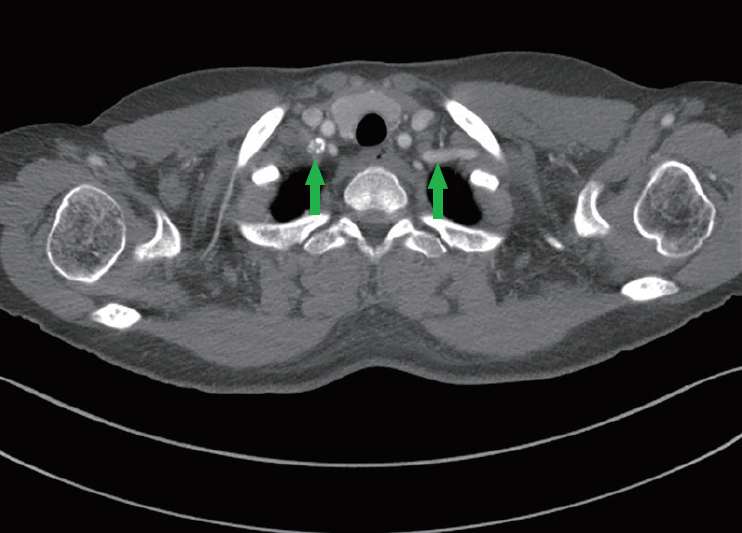
Segmental occlusion of the right subclavian artery
Preoperative computed tomography angiography showed segmental occlusion of the right subclavian artery (arrows) with severe atherosclerosis near the origin of the internal mammary artery.
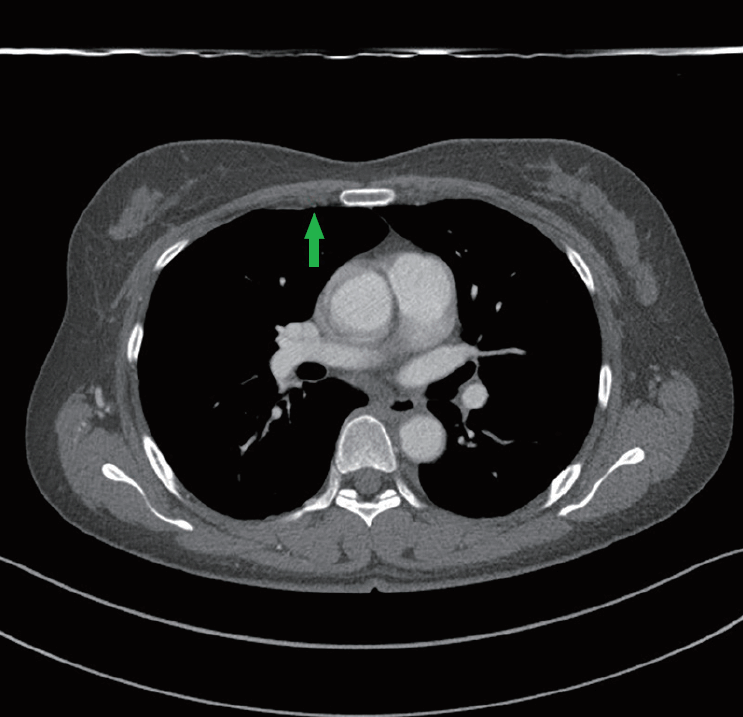
Hypoplastic right internal mammary artery
On preoperative computed tomography angiography, the hypoplastic right internal mammary artery (arrow) was observed at the third intercostal space.
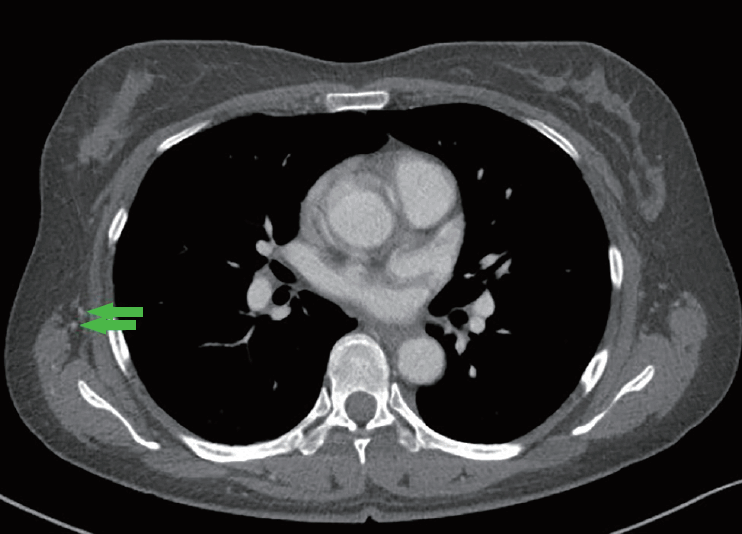
Thoracodorsal artery
The thoracodorsal artery (arrows) showed adequate size and flow on preoperative computed tomography angiography, and was used as the recipient artery for deep inferior epigastric artery perforator flap breast reconstruction.
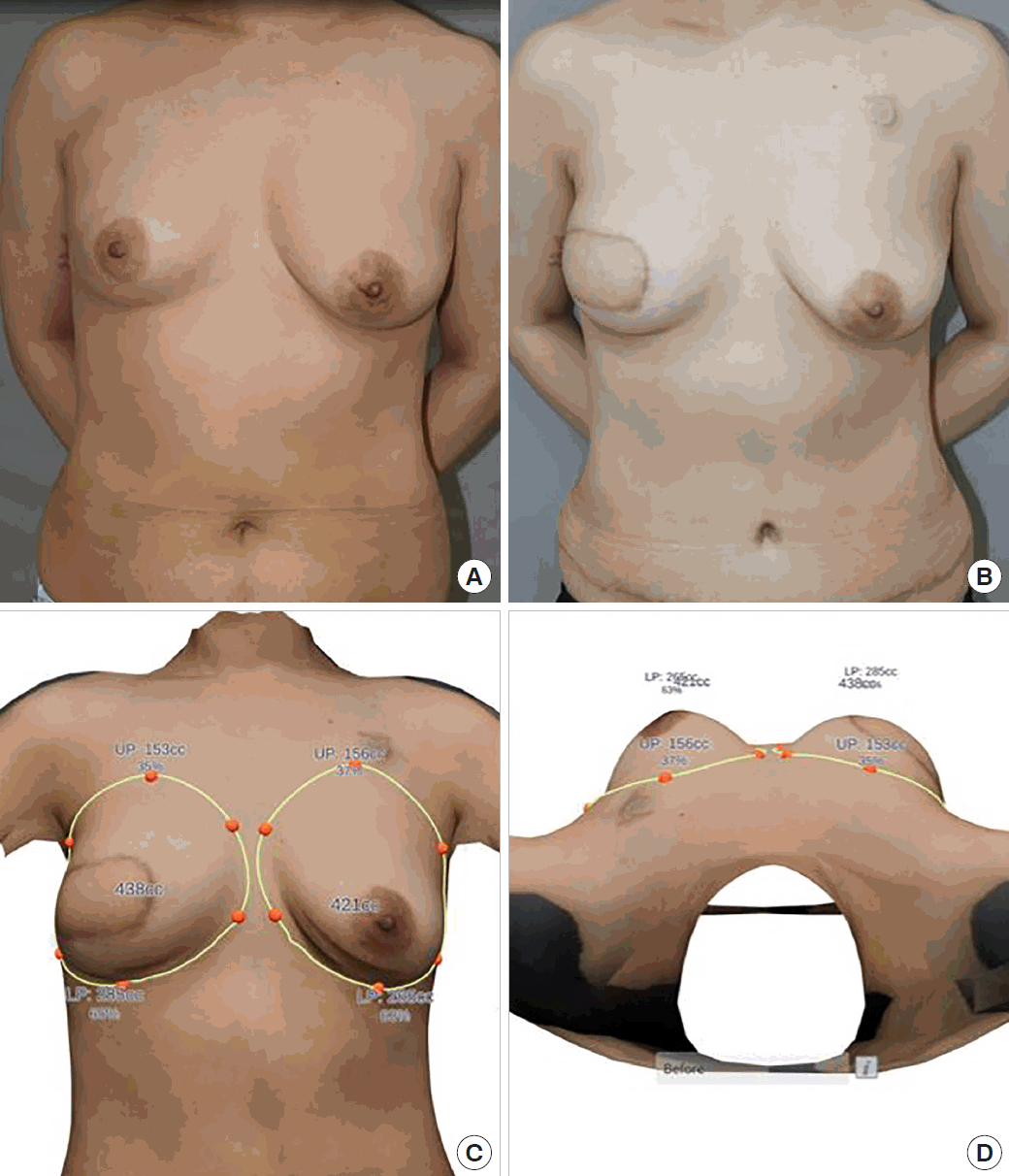
A right skin-sparing mastectomy (360 g) was performed by the oncologic surgery team. A DIEP flap, including three ipsilateral perforators (872 g), was harvested, and the inset rate of the flap was 64% (562/872 g). The pedicle vessels of the flap were anastomosed to the thoracodorsal artery and vein by end-to-end anastomoses. The patient experienced no immediate postoperative complications, and the flap survived without vascular complications. The breasts were symmetrical at a 6-month follow-up and showed no long-term complications. Postoperative three-dimensional photographs at 6 months confirmed symmetrical breast size. The breast volumes were 438 and 421 mL on the right and left, respectively (Fig. 4).
DISCUSSION
The vascular anatomy of the inferior abdominal wall can be evaluated preoperatively with CT angiography [5]. Preoperative CT angiography has been shown to reduce the operative time by minimizing the time spent finding perforators, selecting surgical sites, and identifying the courses of vessels [6]. Three-dimensional CT angiography can be used to estimate abdominal flap volume before surgery, which guides the amount of tissue to harvest [7,8]. Similarly, CT angiography is useful for evaluating recipient vessels [9]. In a study by Sisto and Isola [10], only 3.1% of patients were found to have notable stenosis in the IMA [10]. In addition, Shadman et al. [11] reported that approximately 2% of the independently-living population showed significant subclavian artery stenosis, which was attributed to a previous or current history of smoking, elevated systolic blood pressure, high-density lipoprotein levels (inversely), and the presence of PAD.
In previous studies using the IMA as the recipient vessel for DIEP flaps, IMA sclerosis has not been reported. In a previous study of DIEP flap cases, the IMA was found not to be suitable for vascular anastomosis in three separate cases, and the thoracodorsal artery was instead used. In two cases (one left- and one right-sided reconstruction), the conversion was performed due to friability of the vessel, and in the third case (left-sided reconstruction), the reason for the conversion was absence of the IMA [12].
The current case highlights the fact that the IMA may not always be an appropriate recipient artery because of atherosclerosis and the occlusion of proximal major arteries. Since flow is dependent on a pressure differential, stenosis of the IMA can lead to decreased blood flow and vascular complications of the flap when it is used as a recipient vessel [13]. Of note, Kropf et al. [14] reported a higher rate of fat necrosis after muscle-sparing transverse rectus abdominis myocutaneous flap reconstruction using the thoracodorsal vessels than with the internal mammary vessels. Although the exact mechanism is unknown, lower blood flow of the thoracodorsal artery compared to the IMA may be a factor [15].
The current case report demonstrated that in rare cases, the IMA might be hypoplastic because of proximal atherosclerosis, and that a preoperative CT angiographic evaluation of the recipient vessels in free flap breast reconstruction may be helpful for preventing the use of inappropriate recipient vessels.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (IRB No. 2019-05-024) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Conceptualization: Woo KJ. Methodology: Woo KJ. Data curation: Seong IH. Writing-original draft: Seong IH. Writing-review & editing: Woo KJ.