Surgical management of male genital lymphedema: A systematic review
Article information
Abstract
Genital lymphedema (GL) is an uncommon and disabling disease that manifests as enlargement of the genital region resulting from the disturbance of lymphatic drainage. Although conservative treatment such as decompression is typically the first-line approach, surgical intervention has been shown to be effective in certain cases. This study aimed to systematically review studies evaluating available surgical alternatives for the treatment of male GL. A systematic search strategy using keyword and subject headings was applied to PubMed, Scopus, EMBASE, and Cochrane Library in May 2019. Studies investigating various surgical techniques to treat penile and scrotal lymphedema were included. The potential risk of bias of included trials was evaluated using the methodological index for non-randomized studies (MINORS). In total, 13 studies met the inclusion criteria, nine of which were determined to be high-quality. The average MINORS score was 12.45 for studies involving excision and 14 for studies involving lymphovenous anastomosis (LVA). The most common reason for a low score was a failure to describe the inclusion criteria. Recurrence of lymphedema during follow-up was reported in four studies involving excision and in no studies involving LVA. In general, the quality of the included literature was considered to be fair. Although surgical intervention might not always prevent the recurrence of lymphedema, all of the studies reported improved quality of life after the procedure. This study could be used as the basis for evidence-based guidelines to be applied in clinical practice for managing male GL.
INTRODUCTION
Genital lymphedema (GL) is an uncommon and disabling disease that manifests as enlargement of the genital region resulting from the disturbance of lymphatic drainage. It causes limitations in mobility, voiding, sexual performance, self-hygiene, and social functions. In the United States, GL a most commonly occur as a complication of a malignancy. In contrast, the majority of GL cases in developing nations occur due to infection with Wuchereria bancrofti (filariasis) [1].
Based on its etiology, GL can be considered either primary or secondary [2]. In primary lymphedema, the disturbance of lymphatic drainage may be caused by the obstruction, malformation, or underdevelopment (hypoplasia) of various lymphatic vessels, whereas in secondary lymphedema, acquired diseases may contribute to the disruption or obstruction. Primary lymphedema has various subtypes, which remain poorly understood. The condition can manifest at any age. It is also known as Milroy disease if it occurs in infancy, as lymphedema praecox if it arises during adolescence, and as lymphedema tarda when it appears in patients 30 years of age and older.
A diagnosis can be made from the patient’s history and physical examination, supported by common imaging techniques such as computed tomography, magnetic resonance imaging, and duplex ultrasonography. Currently, the standard tool to evaluate lymphatic function is radionuclide lymphoscintigraphy. It is relatively noninvasive and enables clinicians to make both qualitative and quantitative analyses [3].
Currently, there is no cure for GL, but it can be managed with early recognition and therapy. The standard of care for GL is complex (also known as combined or complete) physical therapy. Conservative therapy may produce good results, but the effects are temporary without maintenance and continued compression. Although conservative treatments such as decompression are the first-line approach, surgical intervention has been shown to be effective in certain cases [4].
Surgical treatments for GL divided into two categories: physiologic and ablative. Physiologic treatments aim to enhance theanterograde flow of lymph by increasing the drainage capacity, while ablative procedures minimize the severity by conducting excision of the affected areas. It is suggested that physiologic treatments should be performed in the early stages as an effort to conserve lymph drainage prior to the progression of fibrosis and adipose deposition. In contrast, ablative treatments are applicable for any stages of lymphedema [5,6].
Currently, the most frequently performed physiologic surgical treatments are lymphaticovenular anastomosis and vascularized lymph node transfer [6]. It is important to underscore the fundamental difference between the older (lymphovenous) and more recent (lymphaticovenular) procedures. In lymphaticovenular techniques, the surgeon identifies patent residual lymphatic channels and performs an anastomosis to a recipient venule. In contrast, in the lymphovenous technique, a recipient vein is used for the anastomosis.
Ablative procedures aim to remove the edematous tissue in order to promote improved function and hygiene. These operations are indicated when lymphedema has progressed to significant fibrosis and fatty infiltration, resulting in the disturbance of the remaining lymphatic channels [6,7]. Debulking or excision techniques for the management of GL can involve partial or total resection of the skin and the subcutaneous tissue associated with lymphatic drainage. The Charles procedure was proposed as a modification of the debulking procedure in which surgeons excise circumferentially and resurface the defects with split-thickness grafts taken from the excised tissue. The Charles procedure is considered to be the earliest and most radical ablative technique [8]. However, the principles remain pertinent to patients with end-stage lymphedema. For select patients, these techniques can offer significant satisfaction and excellent end results. This study aimed to systematically review studies evaluating available surgical alternatives for the treatment of male GL.
METHODS
Search strategy and screening procedure
A comprehensive electronic bibliographic search was performed in May 2019. A gray literature search was not conducted. The electronic databases of PubMed, Scopus, EMBASE, and Cochrane Library were searched using the following medical subject headings (MeSH): “genital lymphedema AND surgical procedure,” “scrotal lymphedema AND surgical procedure,” and “penile lymphedema AND surgical procedure.” Limits applied to the literature search included: “humans,” “English,” “full-text,” and “articles published between 1990 and 2019.”
The titles and abstracts of potentially relevant articles were screened and examined. Lists of references from the articles were also examined to find other relevant studies that were not detected through the electronic search.
Data extraction
The authors reviewed each title and abstract, along with each potentially relevant article. When provided, the following data were extracted: sample population, patient age, etiology, surgical technique(s), early complications, late complications, quality of life, and duration of follow-up. Evaluable studies were analyzed per endpoint.
Quality assessment
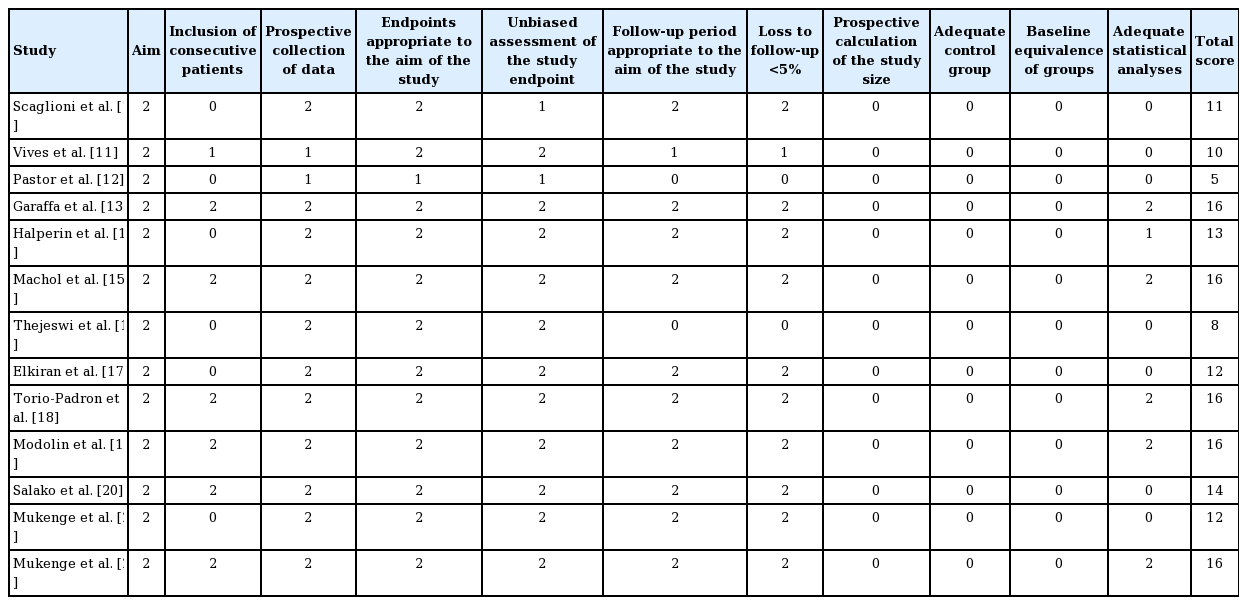
Selected studies were then appraised using the methodological index for non-randomized studies (MINORS), which is a validated instrument used to evaluate the methodological quality of non-randomized surgical studies. Considering the frequent publication of observational studies in surgery, the MINORS score is key in assessing the validity and quality of published data. The MINORS system utilizes an eight-item index (with a global maximum score of 16) for noncomparative studies and a 12-item index (with a global maximum of 24) for comparative studies [9].
RESULTS AND DISCUSSION
Search and screening results
The initial search of PubMed, Scopus, EMBASE, and Cochrane Library using the keywords mentioned above identified 273 relevant studies. After application of the aforementioned limits, only 164 publications remained. Furthermore, 148 studies were excluded after removal of duplicates and screening of the titles and abstracts. Overall, the full texts of 16 articles were examined based on the inclusion and exclusion criteria. Of these, three further articles were excluded: two because the full-text studies were not found and one because it was a conference abstract. A total of 13 studies met the inclusion criteria and were considered eligible for review as described in Fig. 1.
Demographics
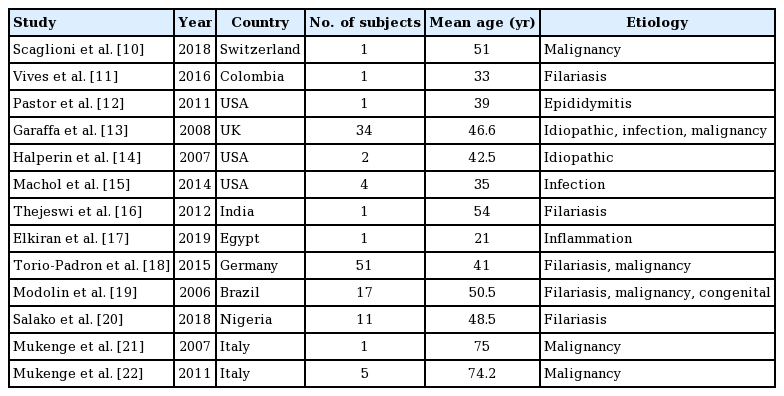
The study populations varied in the characteristics of participants and the sample sizes. The number of participants varied from one to 51 patients who underwent surgical intervention. The patients’ mean age ranged from 21 to 74.2 years.
The studies reviewed were conducted across the globe, with populations of different ethnic backgrounds. The disease underlying GL also varied among the studies. Most cases of GL were due either to filariasis infection or progression of malignancy. However, four studies described idiopathic GL, as shown in Table 1.
Quality of studies
A total of 13 studies met the inclusion criteria. Nine studies were determined to be high-quality, as illustrated in Table 2. The average MINORS score was 12.45 for studies involving excision and 14 for studies involving lymphovenous anastomosis (LVA). The most common reason for a low score was a failure to describe the inclusion criteria. Lymphedema recurrence during follow-up was reported in four studies of excision and in no studies of LVA.
Ablative surgery: Excision and Charles procedure
Excision was the most common surgical intervention found among the included studies. Nine of the reviewed studies illustrated excision procedures to treat GL [10-18]. In contrast, only two studies described the use of modified Charles procedures [19,20].
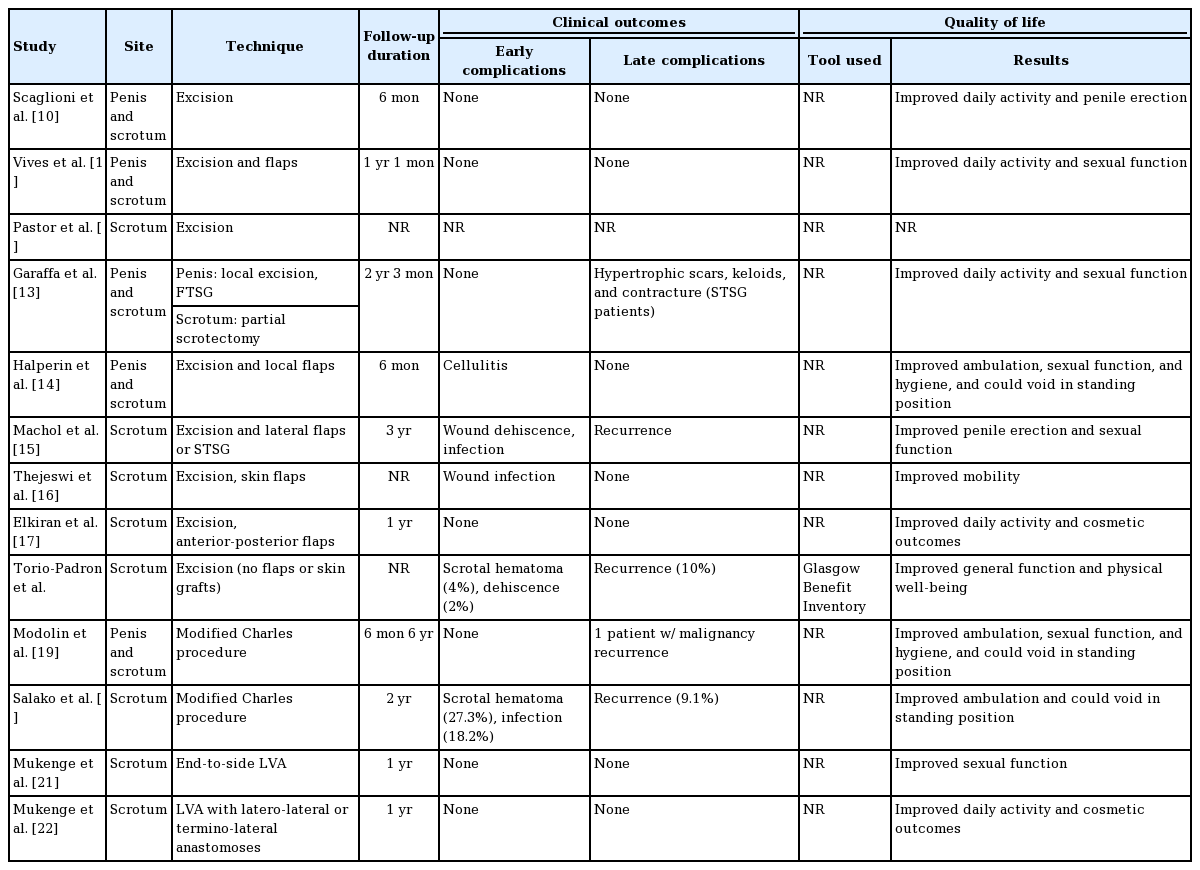
In a study by Scaglioni and Uyulmaz [10], a debulking procedure was followed by a split-thickness skin graft from the unaffected upper thigh to cover the entire penile shaft. In 6 months of follow-up, they did not discover any complication or recurrence. In contrast, Torio-Padron et al. [18] reported early complications such as scrotal hematoma (4%) and wound dehiscence (2%) among a total of 51 patients. However, the follow-up duration was not reported clearly.
Among those studies, some early complications such as cellulitis, infection, wound dehiscence, and scrotal hematoma were reported. In addition, Modolin et al. [19], Machol et al. [15], and Salako et al. [20] illustrated recurrent GL rates of 5%, 25%, and 9.1%, respectively. The earliest disease recurrence was reported to occur during the second year of follow-up.
Physiological surgery: LVA
Despite the fact that lymphaticovenular anastomosis has been widely used for treating GL, several conditions may render cases unsuitable for this technique. Mukenge et al. [21,22] was consistent in treating GL with LVA. Most of the patients included in their studies had been treated for a malignancy prior to complaining of GL. In such severe cases of GL, LVA is considered to be a more effective treatment than lymphaticovenular anastomosis, due to the relatively large diameter of the remaining lymph vessels. Mukenge et al. [21,22] performed LVA between the spermatic funiculus lymphatic collectors and the tributary vessels to the spermatic veins. They conducted end-to-side LVA in their first study and performed latero-lateral or termino-lateral LVA 3 years later as part of a follow-up study. No patients experienced recurrence of the disease during 1 year of follow-up. The authors also described the importance of using the proper method to assess the anastomosis patency during follow-up. In this case, they performed this assessment with noninvasive lymphography using an indocyanine green-photodynamic eye infrared system.
Quality of life
All of the selected studies reported a better quality of life for patients after surgical intervention. Most of the studies evaluated patients’ quality of life by assessing mobility, urinary function, sexual function, and scrotal weight, as depicted in Table 3. However, the measurement of quality of life was not standardized among those studies and seemed to be based on patients’ subjective reports. Most of the studies applied a qualitative approach to follow-up on patients’ functional status. One study adapted the Glasgow Benefit Inventory questionnaire as a quantitative tool for assessing quality of life. Torio-Padron et al. [18] found that the patients had improved general function and physical well-being, while the social support that patients received was unchanged. The selected studies’ results would be more comparable if they had utilized a consistent measurement system, such as the International Classification of Functioning Disability and Health (developed by the World Health Organization), or if they had used quantitative tools that might allow for easier comparison.
LIMITATIONS
Several limitations were encountered in this systematic review, such as a lack of research investigating surgical interventions for the treatment of GL. This may result from the low prevalence of GL cases. In addition, most of the studies did not mention the stage of GL; therefore, we were unable to make a more objective comparison among the results of the selected studies. Moreover, the lack of quality of life measurement tools for lymphedema also allowed us to draw only a subjective conclusion regarding the selected studies.
CONCLUSION
In general, the quality of the included literature is considered to be fair. Even though surgical intervention might not always prevent the recurrence of lymphedema, all of the patients in these studies were found to have a better subjective quality of life after the procedure. Due to the subjective nature of these results, we recommend that future studies utilize more quantitative methods to assess patients’ quality of life. This study could be used as the basis for evidence-based guidelines to be applied in clinical practice for managing male GL.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: Aulia I. Data curation: all authors. Formal analysis: Yessica EC. Methodology: all authors. Project administration: Yessica EC. Visualization: all authors. Writing - original draft: all authors. Writing - review & editing: all authors. Approval of final manuscript: all authors.