Analysis of factors that affect drainage volume after expander-based breast reconstruction
Article information
Abstract
Background
Closed-suction drains are widely used in expander-based breast reconstruction. These drains are typically removed using a volume-based criterion. The drainage volume affects the hospital stay length and the recovery time. However, few studies have analyzed the factors that influence drainage volume after expander-based breast reconstruction.
Methods
We retrospectively analyzed data regarding daily drainage from patients who underwent expander-based breast reconstruction between April 2014 and January 2018 (159 patients, 176 expanders). Patient and operative factors were analyzed regarding their influence on total drainage volume and drain placement duration using univariate and multivariate analyses and analysis of variance.
Results
The mean total drainage volume was 1,210.77±611.44 mL. Univariate analysis showed correlations between total drainage volume and age (B=19.825, P<0.001), body weight (B=17.758, P<0.001), body mass index (B=51.817, P<0.001), and specimen weight (B=1.590, P<0.001). Diabetes history (P<0.001), expander type (P<0.001), and the surgical instrument used (P<0.001) also strongly influenced total drainage. The acellular dermal matrix type used did not affect total drainage (P=0.626). In the multivariate analysis, age (B=11.907, P=0.004), specimen weight (B=0.927, P<0.001), and expander type (B=593.728, P<0.001) were significant predictors of total drainage.
Conclusions
Our findings suggest that the total drainage and the duration of drain placement needed after expander-based breast reconstruction can be predicted using preoperative and intraoperative data. Patient age, specimen weight, and expander type are important predictors of drainage volume. Older patients, heavier specimens, and use of the Mentor rather than the Allergan expander corresponded to a greater total drainage volume and a longer duration of drain placement.
INTRODUCTION
Closed-suction drains are widely used in breast reconstruction. These drains are removed according to a volume-based criterion, which is typically defined as <30 mL/day [1,2]. Although the specific volume criterion used may vary, the general principle is to remove the drain when the drainage volume produced over time decreases. A higher drainage volume results in a longer duration of drain placement and postoperative disruption to patients’ lives. In general, antibiotics are administered as long as a drain remains in place. Depending on the physician’s preference, patients may also be restricted with regard to showering while they have drains in place [1]. Some studies also suggest that longer drainage times increase the risk of surgical-site infections [3-5]. For these reasons, it is of crucial importance for plastic surgeons to be able to predict drainage volume after breast reconstruction.
Several factors may influence postoperative drainage volume. These include patient-related factors (such as obesity and medical history) and operative factors (such as operative time and surgical instruments used) [6]. In expander-based breast reconstruction, the expander type (smooth or textured), the surgical instrument type, and the type of acellular dermal matrix (ADM) may also influence the drainage volume. One study showed that older age, larger breast mass, and axillary lymph node dissection status affected drainage volume [6]. Another study suggested that ADM use was associated with increased drainage and that older age, a larger expander, and a larger drainage volume on the first postoperative day were predictors of a longer time to drain removal [7]. Nonetheless, few studies have analyzed factors that predict drainage volume after breast reconstruction procedures.
In this study, patients’ daily drainage volume following expander-based breast reconstruction was retrospectively reviewed. Additionally, we explored factors that were associated with drainage volume through univariate and multivariate analyses.
METHODS
Patients
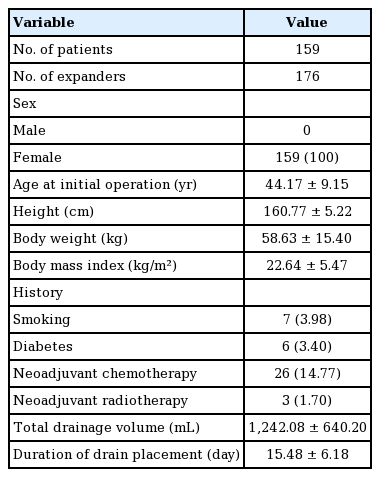
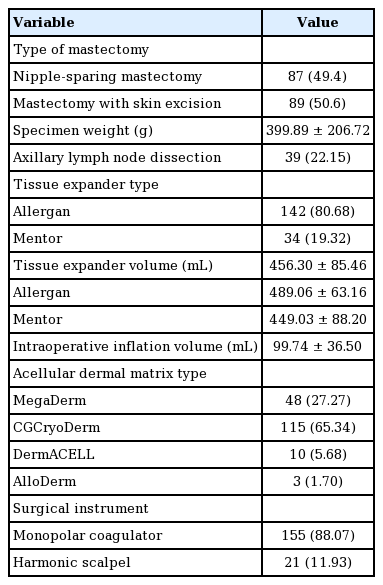
All female patients who underwent expander-based breast reconstruction immediately after mastectomy between April 2014 and January 2018 were retrospectively analyzed. A total of 165 patients were reviewed. Six patients were excluded from the data: one patient with postoperative hematoma, one with postoperative seroma, and four due to a lack of data. After this exclusion, 159 patients and 176 expanders were analyzed. The patient characteristics are shown in Table 1, and the operative factors are summarized in Table 2.
Operative technique
One of three mastectomy techniques was performed: total mastectomy with skin and nipple resection, skin-sparing mastectomy with only nipple resection, and nipple-sparing mastectomy. These types of mastectomies were classified into either mastectomy with skin excision or mastectomy without skin excision. An expander was always used when the skin was removed. Because of the high rate of nipple necrosis in our hospital, two-stage breast reconstruction using an expander is our facility’s default procedure, especially in the cases of patients who request contralateral side breast augmentation. Two types of expanders, the Mentor CPX4 expander (Mentor Worldwide LLC, Irvine, CA, USA) and the Natrelle expander (Allergan PLC, Dublin, Ireland) were used. The type of expander was selected based on the surgeon’s criteria. The width of the chest was the first condition to be considered. After determining the width of the expander, we chose the volume of the expander to achieve the desired breast size. When the patient requested augmentation of the contralateral breast, we selected a high-profile expander corresponding to the target volume. When the patient did not request a contralateral procedure, we selected an expander corresponding to the contralateral breast volume. The expander was placed in the submuscular pocket. The ADM was sutured to the inferolateral border of the pectoralis major muscle and was used to cover the expander. A closed drain was placed in the inframammary fold area, while another was placed in the axillary space. Four types of non-washed, non-frozen ADMs without slit incisions were used: MegaDerm (6×16 cm, 1.5 mm thickness; L&C Bio Corp., Seoul, Korea), CGCryoDerm (6×16 cm, 1–2 mm thickness; CG Bio, Seongnam, Korea), DermACELL (6×16 cm, 1–2 mm thickness; LifeNet Health, Virginia Beach, VA, USA), and AlloDerm (4×12 cm, 2.3–3.3 mm thickness; LifeCell Corp., The Woodlands, TX, USA). Two surgical instruments were used to elevate the pectoralis muscle and to control intraoperative bleeding: a monopolar coagulator, SurgiStat II (Valleylab, Boulder, CO, USA), and an ultrasound surgical device, the Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA).
Postoperative management
Patients were treated with two antibiotics (cephalosporin, 2 g/day and aminoglycoside, 400 mg/day) until the final drain was removed. The drainage volume was checked every day until the removal of the drain. Each drain was removed when its output was <30 mL/day. The duration of drain placement was recorded based on the date when the last drain was removed. The total drainage volume was calculated as the sum of the volumes of both drains.
Statistical analysis
All analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). Data are expressed as the mean± standard deviation. P-values <0.05 were considered to indicate statistical significance. Univariate analyses were performed with each variable. Linear regression was performed between the total drainage volume and each factor as a continuous variable. The B-value refers to how much the dependent variable changes when the independent variable is changed by a 1-unit increment. Comparisons between the two groups were performed using the independent t-test, and comparisons among the four groups were performed using analysis of variance. Factors that showed significant results in univariate analysis were analyzed with multivariate multiple regression analysis. The variance inflation factor was calculated to confirm multicollinearity.
RESULTS
The total drainage volume gradually decreased postoperatively (Fig. 1). The trend for the daily drainage volume over time showed a similar pattern for each expander, but on each postoperative day, patients with Mentor expanders showed more drainage than those with Allergan expanders (Fig. 2). The average duration of drain placement was 15.48±6.18 days, the total drainage was 1,242.08±640.20 mL, and the two were strongly correlated (P<0.001, correlation coefficient=0.892). No statistically significant difference (P=0.086) was found between tissue expander volume according to the type of expander (Mentor expanders, 449.03±88.20 mL; Allergan expanders, 489.06± 63.16 mL) (Table 2).
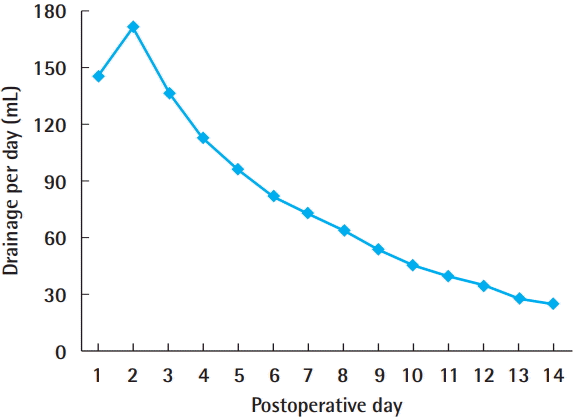
Daily drainage volume over time
Drainage volume was calculated as the sum of the volumes of the two drains.

Daily drainage difference between Allergan and Mentor expanders
Comparison of daily drainage volume over time between patients with Allergan and Mentor expanders. Drainage volume was calculated as the sum of the volumes of the two drains.
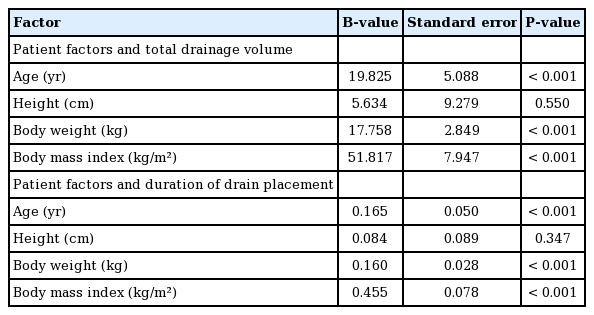
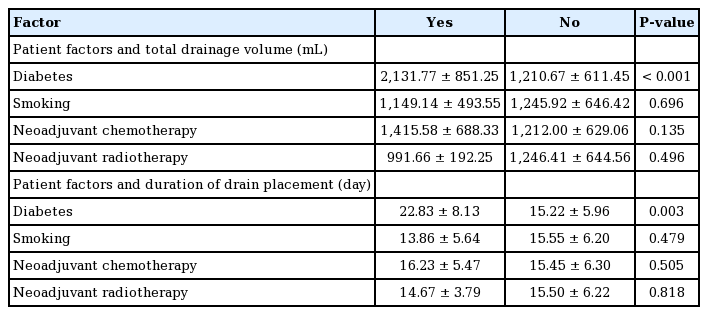
The results of the univariate analysis of patient factors are summarized in Tables 3 and 4. Age (P<0.001, B=0.165) was strongly correlated with total drainage volume. In contrast, height was not associated with total drainage volume (P=0.55). Body weight (P<0.001, B=17.758) and body mass index (BMI) (P<0.001, B=51.817) were strongly correlated with total drainage volume. Patients with diabetes produced a total of 2,131.77±851.25 mL of drainage, while those without diabetes produced 1,210.67±611.45 mL (P<0.001). In contrast, smoking did not have a significant effect on total drainage volume (P=0.696). Patients who received neoadjuvant chemotherapy tended to have a higher total drainage volume than those who did not, although the difference was not significant (P=0.135). In contrast, neoadjuvant radiotherapy did not affect the total drainage (P=0.496).
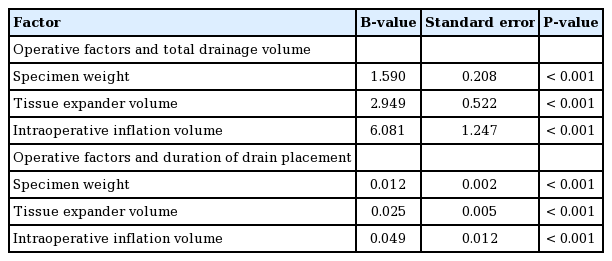
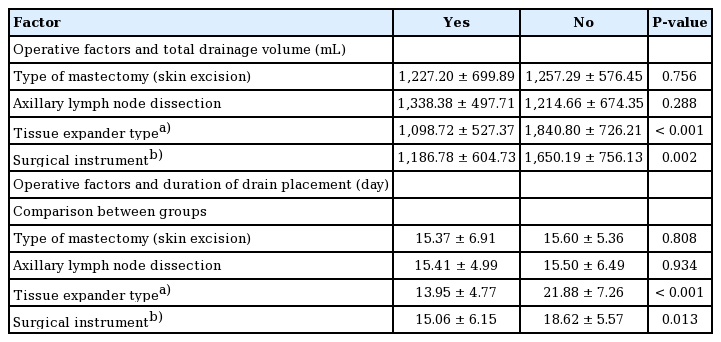
The results of the univariate analysis of operative factors are summarized in Tables 5 and 6. The specimen weight, which was measured immediately after mastectomy, was strongly correlated with the total drainage volume (P<0.001, B=1.590). Other operative factors, including the tissue expander volume (P<0.001, B=2.949) and the intraoperative inflation volume (P<0.001, B=6.081), were also strongly correlated with the total drainage. The mastectomy type (mastectomy with skin excision vs. mastectomy without skin excision) did not affect the total drainage (P=0.756). Axillary lymph node dissection was associated with a higher total drainage volume, but this relationship was also not statistically significant (P=0.288). There were significant differences in the total drainage volume depending on the tissue expander type (Allergan, 1,098.72±527.37 mL; Mentor, 1,840± 726.21 mL; P<0.001). Use of the Mentor expander led to a higher volume of total drainage and a longer duration of drain placement. The type of surgical instrument used had a significant effect on the total drainage volume (monopolar coagulator, 1,186±604.73 mL; Harmonic scalpel, 1,650.19± 756.13 mL; P=0.002). In its relationships with these factors, the duration of drain placement showed largely similar tendencies as the total drainage volume.
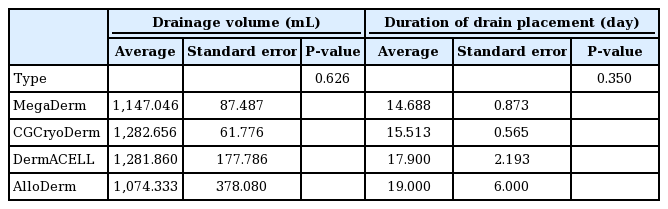
There were slight differences in the total drainage volume and the duration of drain placement according to the type of ADM used (MegaDerm, CGCryoDerm, DermACELL, or AlloDerm), but these differences were not statistically significant (analysis of variance, P=0.626 and P=0.350 for total drainage and duration of drain placement, respectively) (Table 7).
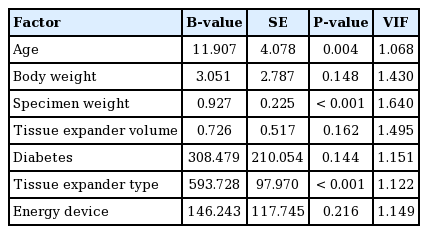
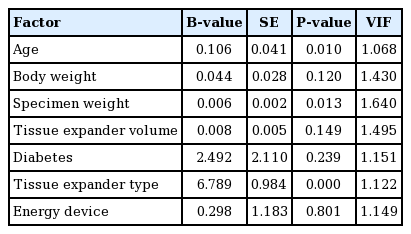
In the multiple regression analyses (adjusted R2, 0.466), age (P=0.004, B=11.907), specimen weight (P<0.001, B=0.927), and tissue expander type (P<0.001, B=593.728) were significantly related to the total drainage volume. Moreover, age (P= 0.010, B=0.106), specimen weight (P=0.013, B=0.006), and tissue expander type (P<0.001, B=6.789) were significantly related to the duration of drain placement (Tables 8 and 9). Patients who were older, had heavier specimen weights, and underwent surgery using an Allergan expander rather than a Mentor expander showed a greater volume of total drainage and a longer duration of drain placement. BMI was significant in the univariate linear regression, but it was excluded from the multiple regression analyses due to the problem of multicollinearity with body weight.
DISCUSSION
It is critical to manage drainage volume after breast reconstruction surgery to prevent complications such as seroma and infection. A randomized study found that closed-suction drainage decreased the incidence and degree of seroma formation [8]. Another study found that a drainage flow rate >50 mL/day was associated with seroma formation postoperatively [9]. Most plastic surgeons use closed-suction drains after breast reconstruction and remove them when the drain output is <30 mL/day [1,2], and we used the same criterion in this study. Only one patient in our study developed a seroma, and the case was excluded from the data. Because a drainage volume exceeding the drain removal standard had continued for more than 6 weeks, the two drains—the drain placed under the inframammary area and the drain placed under the axilla—were not analyzed separately. The total drainage volume and the date of removal of the last drain were recorded.
Age was strongly correlated with the total drainage volume in both univariate and multivariate analyses (P<0.001 and P= 0.004, respectively). A prior study also reported the same result, in that older patients had a higher drainage volume than younger patients [6]. Previous studies of autologous breast reconstruction have found advanced age (>45 years in one study [10] and >65 years in another study [11]) to be a risk factor for seroma formation. Other complications, such as surgical site infection [3] and venous thromboembolism [12], have also been reported more frequently after breast surgery in patients of advanced age than in younger patients.
Specimen weight was strongly correlated with total drainage volume in both univariate and multivariate analyses (P<0.001 and P<0.001, respectively). In the univariate analysis, body weight, mastectomy specimen weight, and BMI were correlated with the total drainage volume, but the multivariate analysis did not find a significant relationship for body weight, mastectomy specimen weight, and BMI. This could have been due to the confounding effect of patients with a higher body weight and BMI having a higher specimen weight. These results suggest that breast mass has a major influence on the total drainage volume after expander-based breast reconstruction, as was previously reported by Suga et al. [6]. The impact of breast mass on outcomes of breast reconstruction have been studied extensively. Woerdeman et al. [13] reported a significantly higher frequency of implant loss in breasts that were larger than average. Duggal et al. [14] found that postoperative wound infections were more common in patients with larger breasts. Furthermore, Wang et al. [15] demonstrated an association of larger breast mass with an elevated risk of superficial nipple necrosis after nipple-sparing mastectomy. In a study of expander-based breast reconstruction procedures, Francis et al. [16] reported that a breast size larger than a C cup was a risk factor for infection. Therefore, patients with large breasts should be informed that they are likely to have a longer duration of drain placement and a higher drainage volume than women with smaller breasts (Tables 5, 6, 8, 9).
The tissue expander type was found to have a significant influence on the total drainage volume after expander-based breast reconstruction in both univariate and multivariate analyses (P<0.001). The group that underwent surgery using the Mentor expander had a higher drainage volume (by approximately 750 mL) than those who underwent surgery using the Allergan expander. This extra volume corresponded to 8 additional days of drainage (Table 6). We used two types of expander, which are manufactured in different ways. The Allergan expander is created using the lost-salt technique, while the Mentor expander is generated using a pressure imprint-stamping technique [17]. The major difference between the two expanders comes from their surfaces. In a recent study, the Allergan implant displayed a larger external surface area per unit of internal surface area than the Mentor implant under microscopy [17]. According to the classification system used by that study, the Allergan implant has a macrotextured surface (200–300 mm2), and the Mentor implant has a microtextured surface (100–200 mm2) [17]. Therefore, we can hypothesize that the difference in surface texturing may relate to the difference in drainage volume. As the number of Mentor expanders used in this study was lower than the number of Allergan expanders (Allergan 142, Mentor 34), it is premature to conclude that the use of a certain implant leads to a greater drainage volume. Nevertheless, this paper is meaningful in that it shows that there may be a difference in drainage volume depending on the surface type of the implant. The surgeon should be aware of the possibility of the difference in postoperative drainage volume depending on the type of implant. However, further research is needed, as there has been no previous research on this topic.
Recently, breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) has become an issue in the use of breast implants. Although the pathophysiology of BIA-ALCL is unclear, a recent report published by the U.S. Food and Drug Administration showed that BIA-ALCL occurs mainly in patients for whom textured implants were used [18]. Disease-specific risks are reported depending on the manufacturer and the implant surface type [19]. The cause of BIA-ALCL is not yet clearly understood. Therefore, multifaceted measurements and analyses of the effect of the implant surface on the human body may help determine the cause of BIA-ALCL. As one of the main symptoms of BIA-ALCL is fluid collection around the implant, it is expected that further studies of the relationship between BIA-ALCL and drainage volume will be of interest and will shed light onto basic aspects of BIA-ALCL.
Patients with diabetes also demonstrated significantly greater drainage volumes than those without diabetes in the univariate analysis (P<0.001). However, this relationship was not significant in the multivariate analysis (P=0.144). Of the six patients with diabetes, one patient was treated with insulin injections, and the other five patients were treated with oral medication. One patient treated with oral medication had normal glucose levels at the day of surgery. In contrast, another study found that a history of diabetes did not impact total drainage [6]. However, that study included only one patient with diabetes, while our study included six patients with diabetes (3.4%). Therefore, more data are needed to clarify these discrepant results.
Patients who were treated with neoadjuvant chemotherapy demonstrated a higher total volume of drainage than those who did not receive neoadjuvant chemotherapy, although the difference was not statistically significant. However, this result may be confounded by whether the patient underwent axillary lymph node dissection. Another study showed that neoadjuvant chemotherapy alone had no effect on total drainage volume [6]. Other studies have reported that neoadjuvant chemotherapy did not increase the complication rate after immediate breast reconstruction [20,21].
Patients who underwent axillary lymph node dissection had higher total drainage volumes than those who did not, although the difference was not statistically significant (P=0.288). A previous study found that axillary lymph node dissection status had a significant influence on total drainage volume following expander-based breast reconstruction procedures [6]. Another study compared short-term and long-term drainage after mastectomy with axillary dissection, and reported a higher incidence of seroma in the short-term drainage group [22]. Furthermore, another study demonstrated an association of axillary dissection with an elevated risk of implant loss in prosthetic breast reconstruction procedures [23]. Therefore, patients who undergo axillary lymph node dissection must be monitored carefully during postoperative management. One effective technique to assess the impact of axillary lymph node dissection would be to measure the amount of drainage from drains inserted into the axillary area independently from other drains inserted elsewhere. Therefore, the fact that the axillary and inframammary drains were not measured separately is a limitation of this study.
Tissue expander volume and intraoperative inflation volume were both strongly correlated with total drainage volume in the univariate analysis (P<0.001). However, this relationship was not significant in the multivariate analysis (P=0.149). This result, however, may have been confounded by the specimen weight, because larger specimens require larger tissue expanders and more intraoperative inflation. With this in mind, when comparing results within the same tissue expander size, no significant difference was found in the drainage volume based on intraoperative inflation volume.
Interestingly, the type of surgical instrument used had a significant influence on the drainage volume in the univariate analysis (P=0.013). However, this relationship was not significant in the multivariate analysis (P=0.216). Patients who underwent surgery using a Harmonic scalpel produced a significantly higher total drainage volume than those who were operated on using a monopolar coagulator. The precise mechanism by which the surgical instruments affect the drainage volume requires further investigation. In addition, the effectiveness of a surgical instrument is greatly influenced by the method and duration of the operation. However, this study did not measure the operation duration or specify the exact operation method. Regardless, surgeons should be aware that there might be differences in drainage volume depending on their choice of surgical instrument.
There were no significant differences between the four types of ADM (MegaDerm, CGCryoDerm, DermACELL, and AlloDerm) with regard to drainage volume or duration of drain placement. This may be meaningful, as no prior studies have addressed this issue. Although there was a difference in the size or thickness of the ADMs used, this difference was not statistically significant.
Similar studies have been performed in the past, but our study is meaningful in that it examined a large number of patients. A previous study showed that age, breast mass, and axillary lymph node dissection status are important factors for predicting the total drainage and duration of drain placement [6]. Our study produced similar conclusions in that age and specimen weight were important predictors. Interestingly, the present study also found that the type of expander had a significant effect on the amount of drainage.
Despite these strengths, this study was limited by selection bias. Patients with known risk factors, such as a history of smoking or diabetes, may have been discouraged from undergoing breast reconstruction. Although criteria for expander selection were specified, surgeons may have suffered from selection bias in the expander selection process. Another limitation of this study is that we did not distinguish between the axillary and inframammary drains with regard to drainage volume. If we had measured the drainage volume of each drain separately, we would have been able to assess the effect of axillary lymph node dissection. The fact that this study was performed by a single surgeon has both advantages and disadvantages. It is effective to compare variables that are not influenced by surgeon-related factors, but it is ineffective to compare variables that are influenced by operative skill, like surgical instrument usage.
Surgeons can better predict the total drainage and duration of drain placement after expander-based breast reconstruction based on preoperative and intraoperative information. For instance, the patient’s age, specimen weight, and expander type used were important factors for predicting drainage volume in this study. In contrast, the drainage volume was not affected by the patient’s height, smoking history, neoadjuvant chemotherapy, neoadjuvant radiotherapy, type of mastectomy, or axillary lymph node dissection.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2018-1195) and performed in accordance with the principles of the Declaration of Helsinki.
Author contribution
Conceptualization: Song SY, Lim YM. Data curation: Lim YM. Formal analysis: Lim YM. Methodology: Song SY. Project administration: Lew DH, Roh TS. Writing - original draft: Lim YM. Writing - review & editing: Song SY, Lim YM. Approval of final manuscript: all authors.