A Rare Case of Abdominal Porocarcinoma
Article information
Eccrine porocarcinoma (EPC) is a cutaneous malignancy that arises from the intraepidermal portion of the eccrine sweat duct (acrosyringium). The eccrine sweat gland is a major sweat gland that is found in virtually all skin. EPC accounts for 0.005% to 0.01% of all cutaneous tumors. It predominantly occurs in elderly people who are more than 60 years old and has a female predominance [1]. As far as we know, only five cases of this medical condition have occurred on the abdomen since Pinkus and Mehregan first described EPS in 1963 [1,2]. Here, we report an unusual case of a 65-year-old female patient with EPC that developed primarily on the abdomen.
In 1992, a 65-year-old female patient presented with a palpable and non-tender mass. No ulceration or any discomfort was noted with respect to the lesion. The mass size was 1 cm×1 cm at the beginning, but the size changed periodically, and the lesion had an oozing serous discharge. There were no other problems. Twenty years later, in July 2012, the mass had undergone rapid enlargement, and the lesion had bloody discharge (Fig. 1).
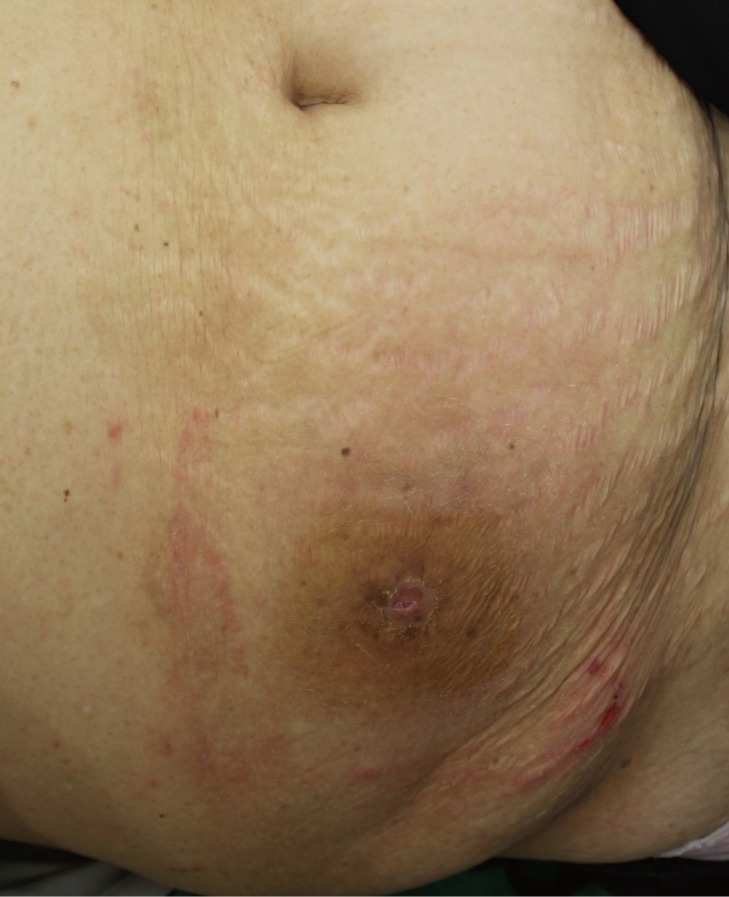
A preoperative photograph shows a brownish maculopapule on the abdomen, and a palpable mass was present.
The patient visited the general surgery department of our hospital. For biopsy, curettage was performed, and a grayish-brown soft tissue was obtained from the skin lesion. The tissue was diagnosed as squamous cell carcinoma. Immunohistochemical staining indicated that the tissue was positive for p63.
The patient was referred to our department for appropriate therapy. Because of the metastatic potential of squamous cell carcinoma, prior to the operation, we performed a physical examination and positron emission tomography-computed tomography (PET-CT) to search for metastasis. No adjacent palpable lymph node was found in the physical examination. PET-CT indicated a hypermetabolic abnormality on the right lower abdomen (Fig. 2), and there was no evidence of metastasis. The laboratory workup and systemic examination for this patient yielded normal results.
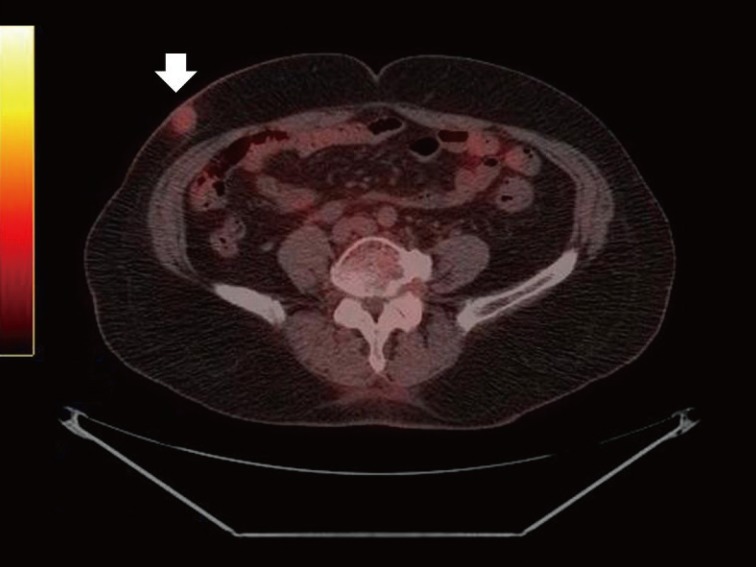
A hypermetabolic abnormality was observed on the right lower abdomen in positron emission tomography-computed tomography (white arrow).
Under general anesthesia, wide excision and frozen biopsy were performed. An approximately 6 cm×3 cm tissue mass was excised, applying at least a 10-mm clear margin. A 1.8-cm well-defined nodule was found within the tissue. Broad tumor free margins were confirmed, and the tumor was diagnosed as EPC by a pathologist. Therapeutic lymphadenectomy was not performed because there was no metastasis according to the PET-CT results. We performed primary repair to reconstruct the soft tissue defect.
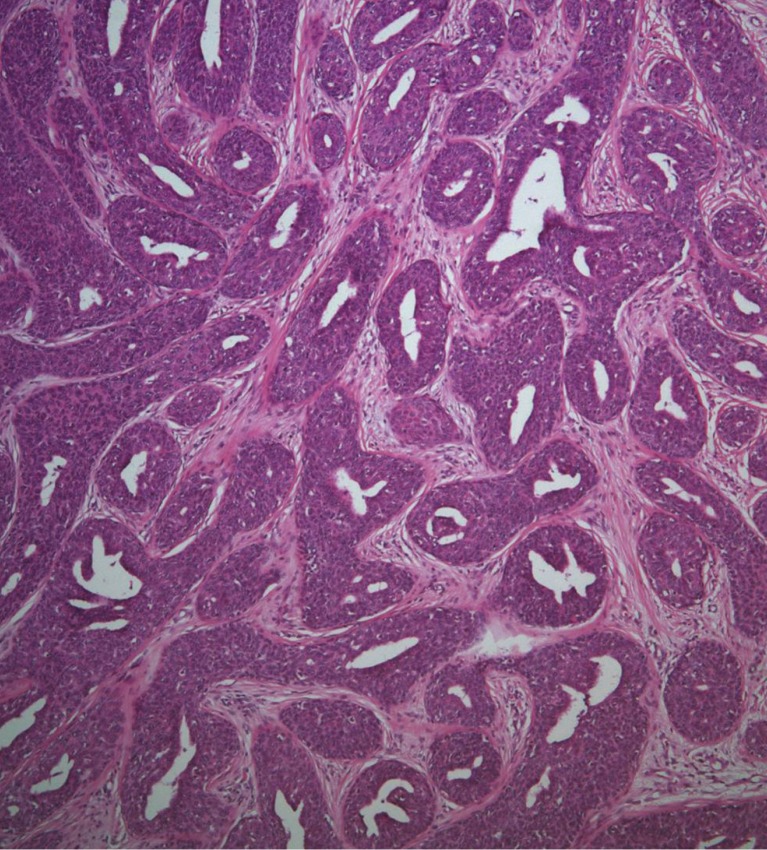
The biopsy histology indicated irregularly proliferating elongated nests composed of small, round basaloid cells and epithelium (Fig. 3), and the tumor nests contained central duct formation (Fig. 4). Immunohistochemical staining was positive for the epithelial membrane antigen (EMA) (Fig. 5), and negative for the carcinoembryonic antigen (CEA); these findings were consistent with a diagnosis of eccrine porocarcinoma [2].
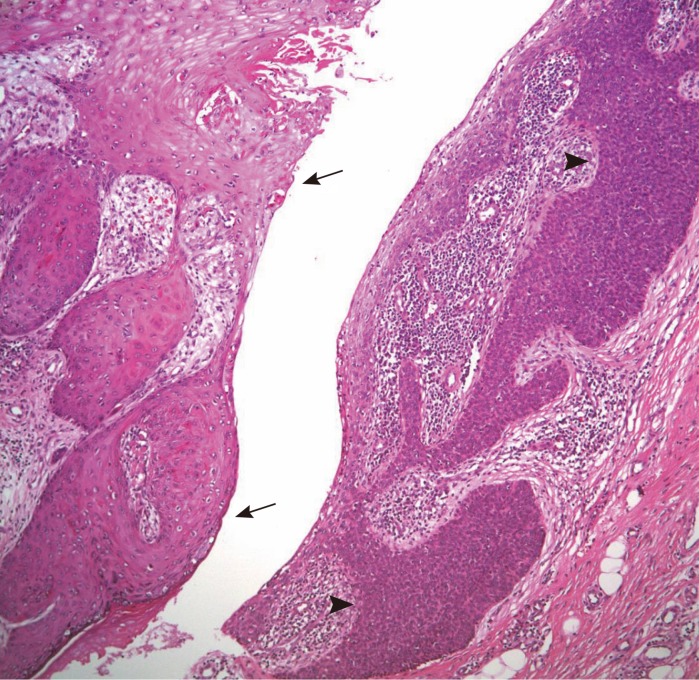
The right-side epithelium shows irregularly proliferating elongated nests composed of small, round basaloid cells (black arrow heads). The left-side epithelium reveals a complex structure of keratinizing squamous epithelium (black arrows) (H&E, ×100).
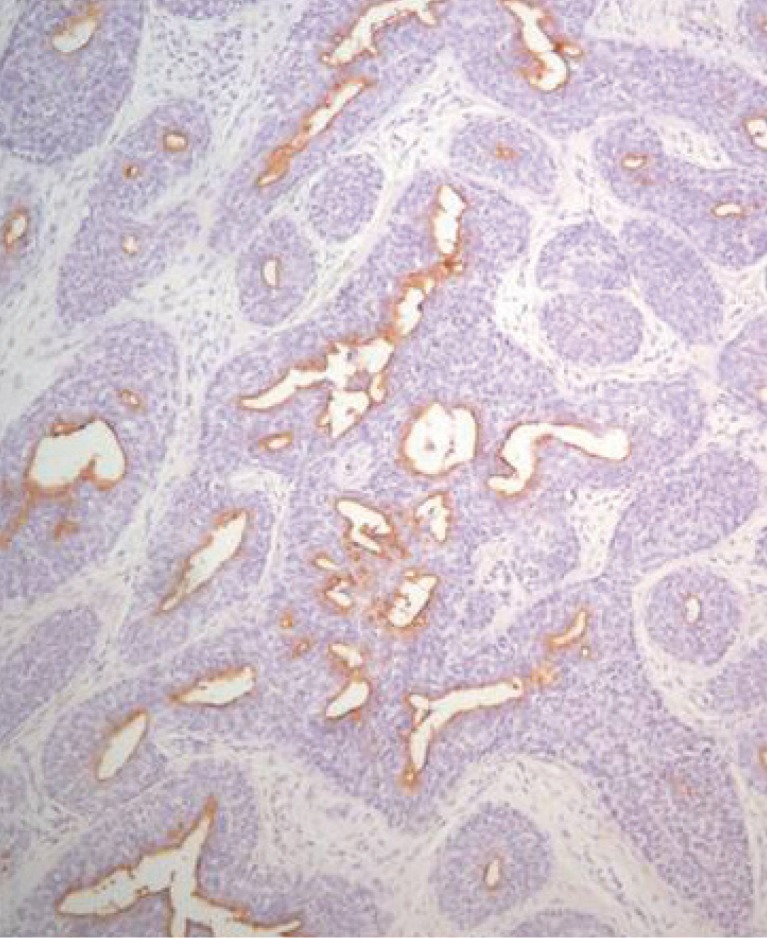
An immunoperoxidase stain is positive for epithelial membrane antigen, suggesting eccrine differentiation.
The patient was followed up at our department because of the high recurrence of EPC. At 7 months after the operation, there was no evidence of recurrence or metastasis.
EPC has a propensity to form on the lower limbs (44%), trunk (24%), head (18%), upper limbs (11%), and neck (3%) [2]. Morphologically, the tumors vary greatly in size, from less than 1 to 10 cm [3].
A long period of clinical features is historically noted (up to 50 years) because some of these tumors can arise from a benign eccrine poroma.Local and regional lymph node metastasis are observed in approximately 20% of patients, and distant metastasis, for example, visceral and bone metastasis, arise in approximately 10% of the patients [3]. Following metastasis, the prognosis is poor, with a reported mortality rate of up to 80% [1]. Because EPC has a high metastatic potential and mortality rate, early definitive diagnosis is necessary, followed by appropriate treatment.
The tumor may appear nodular, infiltrative, ulcerated, or polypoid, and these characteristics tend to indicate a malignant tumor. Multinodularity, ulceration, and rapid growth may be associated with either local recurrence or metastasis [3]. Thus, when these characteristics are observed, we need to consider the possibility of a malignant change.
Because of the rarity and nonspecific appearance of EPC, a tentative clinical diagnosis will never be accurate and the condition might be misdiagnosed as squamous cell carcinoma, basal cell carcinoma, seborrheic keratosis, or metastatic adenocarcinoma [3]. In particular, as in our case, a small biopsy specimen, such as a needle or punch biopsy, can be easily misdiagnosed. Even defined EPC may present some type of basal or squamous differentiation. The presence of ductal differentiation distinguishes EPC from squamous and sebaceous cell carcinoma. The ducts are usually visible in a microscopic evaluation and can be outlined with CEA and EMA immunohistochemical markers [4]. Further, most metastatic adenocarcinomas are positive for CEA and EMA stains. However, CEA is negative in most EPCs but can be positive in tumors containing well-formed ducts [2]. To achieve a definitive diagnosis, a well-handled specimen and expert pathology and oncology departments are required.
Histological findings that predict the worse prognosis are lymphovascular invasion, a mitotic index of more than 14 mitotic cells for every high power field, and a tumoral depth of more than 7 mm [2,4,5].
There is no standard therapeutic protocol for EPC. However, because of its high rate of local recurrence, wide excision with histologically clear margins is the treatment of choice. Appropriate surgical resection achieves curative outcomes in 70% to 80% of the cases, and there is 20% local recurrence and 20% distant metastasis [5]. Because of the high recurrence rate, close follow-up and a search for metastasis should be performed, and sometimes, adjuvant therapy could be performed. However, anecdotal reports show some benefits when utilizing radiation or chemotherapy [3].
EPC is curable if an accurate diagnosis is performed early and appropriate treatment is provided. EPC can be easily misdiagnosed because of a lack of particular characteristics; therefore, a careful approach is necessary. Because of its aggressive potential for metastasis and poor prognosis, a complete early definitive pathologic diagnosis of EPC should be performed along with regional lymph-node involvement assessment, such as a physical examination, and radiological tests. PET-CT can easily indicate whether metastasis is present. Thus, prior to any operation, a PET-CT scan should be performed, as it can assist in determining whether selective adjacent lymph node resection is required.
Notes
No potential conflict of interest relevant to this article was reported.