The Clinical Implications of Poly Implant Prothèse Breast Implants: An Overview
Article information
Abstract
Mammary implants marketed by Poly Implant Prothèse (PIP) were found to contain industrial grade silicone and this caused heightened anxiety and extensive publicity regarding their safety in humans. These implants were used in a large number of patients worldwide for augmentation or breast reconstruction. We reviewed articles identified by searches of Medline, PubMed, Embase, and Google Scholar databases up to May 2014 using the terms: "PIP", "Poly Implant Prothèse", "breast implants" and "augmentation mammoplasty" "siloxanes" or "silicone". In addition the websites of regulating bodies in Europe, USA, and Australia were searched for reports related to PIP mammary implants. PIP mammary implants are more likely to rupture than other implants and can cause adverse effects in the short to the medium term related to the symptoms of rupture such as pain, lumps in the breast and axilla and anxiety. Based on peer-reviewed published studies we have calculated an overall rupture rate of 14.5% (383/2,635) for PIP implants. However, there is no evidence that PIP implant rupture causes long-term adverse health effects in humans so far. Silicone lymphadenopathy represents a foreign body reaction and should be treated conservatively. The long-term adverse effects usually arise from inappropriate extensive surgery, such as axillary lymph node dissection or extensive resection of breast tissue due to silicone leakage.
INTRODUCTION
The recent controversy surrounding the use of industrial grade silicone in mammary implants marketed by Poly Implant Prothèse (PIP) has caused concern among patients and physicians. By 2012, implants manufactured by PIP were found to be associated with failure rates in excess of other implants in several studies. There is much uncertainty regarding the mid-term and long-term consequences of this debacle. In addition to product recalls, many patients are considering replacement of the implants, potentially increasing their risk due to additional surgical procedures [1].
In this study, we intend to review the published literature to assess the long-term consequence of this affair, and the potential implications for patients exposed to these implants.
For this purpose, we identified articles by searches of Medline, PubMed, Embase, and Google Scholar databases up to March 2014 using the terms: "PIP", "Poly Implant Prothèse", "breast implants" and "augmentation mammoplasty", "siloxanes" or "silicone". In addition the websites of regulating bodies in Europe, USA and Australia were searched for reports related to PIP mammary implants.
PHYSICAL AND CHEMICAL PROPERTIES OF PIP IMPLANTS
The primary concern regarding PIP implants has been their higher than expected risk of rupture compared to market competitors [2].
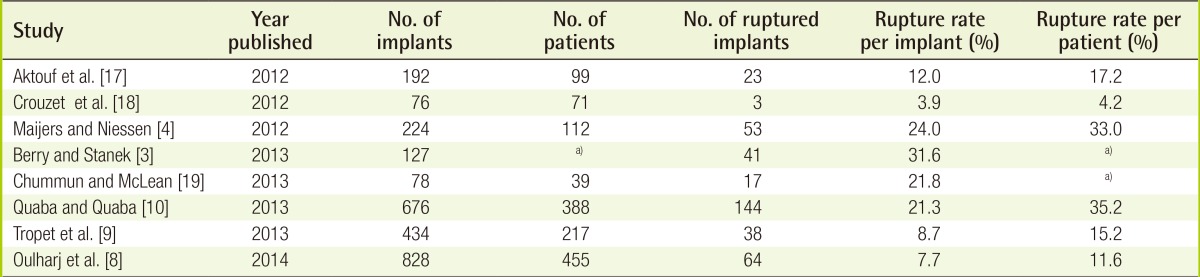
However, the reported rate of rupture varies from one study to another depending upon the methodology and rupture definition used. Berry and Stanek [3] reported a 10 year approximate rupture rate of 30% for PIP implants whereas Maijers and Niessen [4] reported a 10 year failure rate of 24%. The Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM) [5] reported a 16.6% rupture rate after an average implantation period of 5-6 years. These figures compare with an 8-10 year rupture rate of 1.7%-15% for non-PIP implants [6,7].
Since the PIP publicity, a more recent study by Oulharj et al. [8] of 828 explanted PIP implants for curative and preventative reasons reported a lower rate of rupture (7.7%) than earlier studies. A similar rupture rate (8.7%) was reported by Tropet et al. [9] in a series of 434 explanted PIP implants.
Quaba and Quaba [10] reported a failure rate of 21.3% per implant in 388 patients over 8 years, with a significant increase in the rate of failure after 2003.
The 6 year rupture rate for fifth generation silicone implants (considered to be the best available implants) has been reported to be 2% for Mentor memory gel [11] and 3.8% for Natrelle implants [12]. The involvement of the implants manufacturers in such publications represents a potential source of bias.
It should be highlighted that there are several limitations for the reports related to the rupture of PIP implants due to the fact that many of these studies were based on data generated by the recall following adverse publicity and were not prospectively collected. In fact, the adverse publicity was initiated by case reports in 2007 [13,14]. This underscores the need to adopt uniform definitions for mammary implant rupture and failure, as well as a centralised system for reporting such events, in order to help healthcare providers and patients to make informed decisions. Indeed, the maintenance of an effective breast implant registry was one of the key recommendations in the Howe Report regarding the actions of the UK Medicines and Healthcare products Regulatory Agency (MHRA) during the PIP crisis [15].
Furthermore, much of the explantations were driven by concerns regarding the implants rather than clinical signs or symptoms. In a review of 224 patients, Maijers and Niessen [16] found no evidence of an association between clinical symptoms and implant rupture.
Based on peer-reviewed published studies we have calculated an overall rupture rate of 14.5% (383/2635) for PIP implants (Table 1) [3,4,8,9,10,17,18,19]. In contrast, current generation Natrelle implants (Allergan) have a reported 10-year implant rupture rate of 7.7% [20].
There remains significant ambiguity in the published literature regarding the specific flaw in the implants that lead to their failure. This is in part due to a lack of readily accessible information regarding the specific processes used in the manufacture of the PIP implants. Several studies have attempted to delineate the specific flaws in the devices, and extrapolate the points at which quality control may have failed.
In a study comparing 19 explanted ruptured PIP implants with two new implants, Swarts et al. [21] found areas on the surfaces of nearly all the explanted devices where the absolute minimum thickness of the shell was below 0.57 mm. This was below the manufacturer's specified range of 0.57-0.95 mm. As these finding pertained to macro-textured implants, the authors attributed their findings to deficiencies manufacturing techniques specific to such implants [21]. The manufacture of textured implants may require finishing by hand dipping in silicone and pushing on to a bed of salt ('lost-salt' process) before curing [22]. The authors suspected a lack of quality control at this stage of manufacture [21].
Yildirimer et al. [23] compared 18 explanted PIP implants with four controls. Specifically, the devices were subjected to mechanical stress testing and spectroscopy. They found PIP silicone shells to have significantly weaker mechanical strength compared to controls. Furthermore, spectroscopy demonstrated changes suggestive of degradation of the Si-O-Si cross-links of the silicone in the PIP shells to Si-OH. This change correlated to the duration of implantation. A co-existing 'protein-like' spike in the spectroscopy suggested that this degradation may be due to the presence of a bio-film, which may be related to inflammation [23].
Finally, Beretta and Malacco [24] compared the filler materials of a ruptured explanted PIP device with a virgin implant (McGhan) and a sample of technical-grade silicone using rheological techniques, attenuated total reflectance infrared spectroscopy, nuclear magnetic resonance, gas chromatography coupled to mass spectrometry and flow injection electrospray mass spectrometry. They found a lack of cross-linkages in the gel from the PIP device, which corresponded with the lack of cohesiveness compared to medical-grade silicone. Furthermore, they also found significant amounts of cholesterol, which they believed was due to defects in the elastomer shell of the PIP implant [24].
In summary, the most relevant flaw identified in the case of the errant PIP devices has been the strength of the shell rather than the quality, or lack thereof, of the filler. Issues regarding the integrity of the shell of PIP implants were noted even before the introduction of the sub-standard filler gel [4,21]. In other words, the use of the sub-standard silicone may have not been directly relevant to the specific issue of higher than expected rupture rate in PIP implants, even if it was indicative of a criminal lack of quality control and a failure of regulation.
TOXICITY
There is no evidence of any significant organic or inorganic chemicals present, other than siloxanes. However, PIP implant's silicone gels contain significantly higher levels of low-molecular weight cyclic silicones (dimethyl siloxanes), namely octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5) and dodecamethylcyclohexasiloxane (D6), compared with medical-grade silicone implants. The levels of these Siloxanes are higher in macro-textured PIP implants, compared with micro-textured implants [25,26].
These low molecular-weight siloxanes are widely used in cosmetics, food products and domestic products. Therefore, human exposure to these chemicals is almost universal and all women, regardless of whether or not they have breast implants, are likely to have measurable levels of D4, D5, and D6 in their tissues [27]. D4, D5, and D6 do not readily pass biological membranes and, therefore, tend to accumulate in the fluid surrounding the implants. The molecules which pass the fibrous capsule surrounding the implant are cleared via the blood and lymph or, because of their lipophilic nature, tend to be deposited in local fatty tissue in the breast. The metabolic fate involves oxidation to more polar and water soluble metabolites. Urinary excretion is the main route of clearance for water-soluble metabolites and exhalation is an important route of clearance for D4 and D5 [28,29,30].
The various studies commissioned by the regulatory bodies in 2010 and 2012 have confirmed that PIP silicone does not have any genotoxic potential. Furthermore, there is no evidence that the silicone used in PIP implants is immunotoxic [25]. Although the initial testing by AFSSAP in 2010 showed positive skin irritancy for PIP silicone [31], subsequent investigations by the Australian Therapeutic Goods Administration (TGA) in Australia and Europe and by the MHRA in the UK demonstrated that PIP silicone lacked skin irritant activity. The irritancy tests for D4, D5, and D6 were also negative.
There is no evidence that chronic human exposure to siloxanes at levels similar to those found in women who had a rupture of PIP implants is carcinogenic [25,32]. Furthermore exposure to PIP implants does not seem to increase the risk of breast cancer or anaplastic large cell lymphoma (ALCL) [33]. There is, however, limited evidence that chronic exposure to D5 at very high levels might increase the risk of developing uterine polyps and uterine cancer in animals [29]. This level of exposure is much higher than in women who had rupture of PIP implants.
D4 has weak estrogenic activity. Studies in rats exposed to whole body vapour have demonstrated that D4 may affect mammalian fertility, with a no observed adverse effect concentration (NOAEC) of 300 parts per million (ppm) [34]. On the basis of this study, D4 has been assigned a H361f hazard statement denoting that it is suspected of damaging fertility [35]. This has been cited by some authors as a cause for concern in relation to PIP implants, who alluded to the evidence of human toxicity regarding bisphenol A, a substance with similar hazard classification with better documented effects on human fertility [36]. Indeed, periprosthetic fluid near PIP implants have been found to have D4 concentrations of 0-261 ppm (median=136), which was far in excess to what was seen in other implants [37]. It has to be emphasised that the long-term effect of siloxanes, including D4, in humans is unknown. They have been extensively used in cosmetics for the last two decades. Furthermore, extensive evidence has been found of accumulation and persistence of D4 in the environment. However, evidence of any ill-effects in humans is currently unavailable [28,35]. Finally, the expected level of exposure to D4 in patients with PIP implants, whilst in excess of levels seen with other silicone implants, is well below the NOAEC set by the murine study that prompted the issuance of the aforementioned hazard statement.
Therefore, there is no clear evidence that women who had rupture of PIP implants are at higher risk of adverse health events than those who had rupture of non-PIP silicone implants [38,39].
LIFE SPAN AND RE-AUGMENTATION
As is the case with all other breast implants, PIP device would require removal or replacement towards the end of their life span. Therefore, we believe it is worth briefly discussing the relevant clinical considerations regarding re-augmentation and removal.
It is usual to explain to patients that the average lifespan of current breast implants is 10-15 years. Excluding any medical complications, such as infection and haematoma formation, the reasons for re-operations in women who have primary breast augmentation include capsule formation around the implant leading to hardening and change in the shape of the breast, implant rupture and aesthetic revision. The latter category relates to issues such as asymmetry, mal-positioning, rippling, palpability of implant, the patient's preference to change the size of the implant and the development of ptosis with aging. The development of capsule formation is the commonest cause for re-operation and this tends to occur after an average period of 10 years following the primary breast augmentation. It is worth noting that there is currently no evidence to suggest that PIP implants are more or less prone to capsule formation compared to other devices. Overall, approximately 30% of women undergoing primary augmentation require one additional surgical procedure within the first six years and those who have surgical revision are more likely to require further surgical revision in the future for both medical and aesthetic reasons [40].
A very small percentage of women request removal of the implants due to the development of other health issues, such as autoimmune disorders and they assume that the implants are the cause, despite the fact that there is no evidence from large epidemiological studies. Breast implants are not lifetime devices and women should expect at least one procedure to replace or remove them during their lifetime. The number of procedures required in the future largely depends upon the age of the patient at the time of initial augmentation.
CLINICAL CONSIDERATIONS
Our own clinical observations of a higher incidence of implant rupture in women who had PIP implants compared with women who had other types of implants (unpublished) are consistent with the literature [38]. Most cases of PIP implant rupture were clinically silent and the patient did not report any symptoms but the rupture was detected on ultrasound scanning of both breasts and axillae. Symptoms related to rupture of PIP implants include the development of palpable lymph nodes in the axillae, change in the consistency and shape of the breast, development of a breast lump and, less commonly, pain.
Magnetic resonance imaging (MRI) is currently considered the gold standard to confirm the diagnosis of implant rupture with a sensitivity and specificity that exceed 90% [41]. However, due to costs this investigation is not performed in all patients and therefore, doctors tend to rely on ultrasound scanning of both breasts and axillae, which is adequately accurate and is significantly cheaper than MRI. In particular, the ultrasound scan appearances of lymph nodes containing silicone particles or breast lumps due to silicone leak are usually characteristic. However, a significant number of women have requested removal or replacement of the PIP implants due to the anxiety related to the media reports and have had no local symptoms related to the implants. It has been reported that PIP implants are softer and more likely to have yellow discolouration than other implants. This observation, which is not unique to PIP implants has been attributed to higher liquidity of the silicone and a higher tendency of cholesterol absorption into the implants (Fig. 1). In addition to observing excessive sweating (gel bleed), complete disruption of the shell is more common with PIP implants than other implants and the fluid surrounding the ruptured implant is more frequently cloudy and turbid in appearance [14].
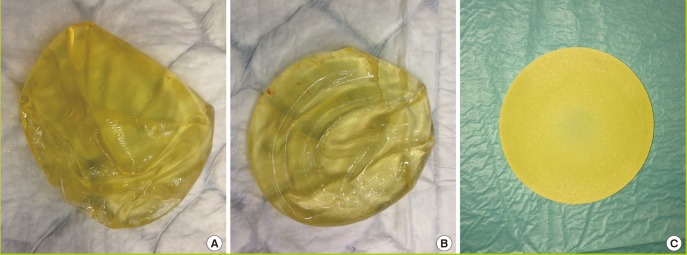
Comparison of replaced PIP and non-PIP implants
(A, B) Poly Implant Prothèse (PIP) silicone implants showing deformation and ruptures. It has been noted that PIP implants require replacement due to failure after 5-6 years. (C) Intact non-PIP implant (Allergan) with yellow discolouration after elective replacement 6 months after implantation.
Furthermore, the incidence of silicone lymphadenopathy is higher in women who had PIP implants due to the higher incidence of rupture [42]. However ruptured PIP implants are treated in the same way as ruptured non-PIP implants and the treatment usually consists of evacuation of the free silicone in the pocket and, if a significant capsule is present, this is treated with either total or partial capsulectomy or capsulotomy depending upon severity (Baker's grade) and clinical indications.
Capsular contracture, which is multi-factorial in aetiology, remains the leading indication for revision of surgery following primary breast augmentation. This complication can occur after augmentation, regardless of the type of implant used. It is unclear whether the incidence of capsular contracture is higher with PIP implants compared with other implants, in view of the higher rupture rate. Since leaking silicone particles are not confined to the capsule, and have been detected in the breast tissue and the tissue of other organs, such as fat and muscle [43], there the capsular contracture associated with PIP implants should be treated in a similar manner to that related to non-PIP implants. Furthermore, there is no correlation between the severity of inflammatory reaction and concentration of silicone particles found in human tissues [27].
If there is localised leak causing a discrete breast lump, associated with an inflammatory reaction, then the lump can be excised for histological examination at the time of evacuation of the free silicone. However, conservative management is recommended for lymph nodes containing silicone particles, since removal of such lymph nodes is associated with a significant morbidity to the patient [42].
The diagnosis of silicone lymphadenopathy is easily established using ultrasonography by an experienced breast radiologist and, in doubtful cases, fine needle aspiration cytology under ultrasound guidance is performed in order to confirm the clinical diagnosis and differentiate it from cancer. In clinical practice patients who receive conservative management for silicone lymphadenopathy recover well following adequate reassurance. Extensive resection of breast tissue is not recommended in cases of multiple areas of silicone leak, since this is associated with an increased surgical morbidity and aesthetic compromise. It should be explained to patients that there is no need to evacuate every silicone particle, since the body slowly deals with these silicone particles through metabolism and excretion and that these particles are present in other organs and therefore it is not possible to evacuate every silicone particle by surgery. In fact, silicone particles are found in human body tissue even in individuals who have never had silicone implants due to environmental exposure. Certainly, there is no indication for preventative mastectomy, even in cases of extensive leakage within the breast tissue or to reduce the risk of breast cancer.
Patients undergoing removal or replacement of ruptured PIP implants recover quickly from the surgical procedure which is usually performed as a day surgery case or an overnight stay in hospital. They are usually fit to return to work and normal daily activities within one week. Their physical recovery is similar to those who had surgery for ruptured non-PIP implants and their prognosis is normal.
Despite the heightened anxiety and extensive publicity regarding PIP silicone implants, there is no evidence that PIP implant rupture causes adverse health events in humans. The long-term adverse effects usually arise from unwise extensive surgery, such as axillary lymph node dissection or extensive resection of breast tissue due to silicone leakage. Women who have surgical revision following breast augmentation are more likely to undergo further re-operations in the future. This is can be related to a higher risk of complications such as capsule formation [40] or to the psychological profile of the patient.
CONCLUSIONS
There is clear evidence that PIP implants have not been manufactured to the acceptable standard and, therefore, they are associated with a higher risk of rupture than other implants. There is, however, no evidence so far that the industrial grade silicone used in these implants has significant adverse health effects in humans to our knowledge. Women who had these implants are more likely to have revision surgery to have the implants removed or replaced due to implant rupture. Surgeons and health care providers used these implants in good faith on the basis that they met the acceptable standards due to approval by the relevant regulatory bodies. It was not feasible to detect the substandard quality of these implants at the time of implantation on the basis of macroscopic inspection and handling. This crisis has prompted extensive reviews of regulatory frameworks in place for medical devices in several jurisdictions [5,15,44].
The preponderance of the extant evidence precludes any ill effects in the exposed patient population other than those attributable to the unacceptable rate of rupture in the devices in question. The clinician should assist the patient to judiciously and cautiously weigh the theoretical risk attendant to retaining such a device against the very real risks of complications due to prophylactic explantations. Scarring due to avoidable surgical procedures may lead to more significant predictable morbidity than what could be attributed to retaining the PIP implants. It may be more prudent to regularly screen the affected patients for implant rupture with annual clinical examinations and MRI scans, and proceed with further management as would be appropriate. However, if such measures do not assuage the patient's anxiety regarding these devices, offering explantation before the detection of an actual rupture may not be unreasonable, as a high degree of certainty cannot be claimed regarding the sequelae of this evolving scandal.
Notes
This study was funded by grants from the Breast Cancer Hope Foundation (London, UK).
No potential conflict of interest relevant to this article was reported.