Simultaneous Reconstruction of Forefoot and Hindfoot Defects with a Thoracodorsal-Axis Chimeric Flap
Article information
To achieve successful results in foot reconstruction, both form and function should be restored. The reconstruction of soft tissue should be durable against pressure and permit the patient to wear near-normal shoes. However, resurfacing plantar soft-tissue defects is often challenging for reconstructive surgeons because the soft tissue is not redundant, and the plantar skin is characteristically thick, which prevents stretching from occurring easily. Therefore, free-flap surgery is usually needed to resurface moderate-to-large defects [1].
Furthermore, multiple free-flap procedures might be needed to repair multiple large plantar defects. Here, we present a case with large soft-tissue defects in the patient's forefoot and hindfoot that were simultaneously reconstructed using a thoracodorsal-axis chimeric flap. To the best of our knowledge, this is the first case in which we can reconstruct dual defects by using a single microsurgical procedure and preserve the normal foot shape, particularly in the plantar arch and the weight-bearing area.
A 57-year-old woman with a 10-year history of diabetes mellitus (DM) visited the emergency room for severe swelling, pain, and bullae that had formed in her right foot after a long walk 10 days before her visit. On the physical examination, necrotic skin changes were observed in the right forefoot (6 cm×4 cm) and hindfoot (8 cm×4 cm). Severe erythema and swelling were also present around the hindfoot and midfoot areas.
Emergency debridement was performed, and an extensive abscess pocket and necrotic changes were observed in the subcutaneous layer and the plantar aponeurosis of the hindfoot. In the forefoot defect, the soft tissue was necrotized deep to the periosteal layer of the third and fourth metatarsals (Fig. 1). Pseudomonas aeruginosa and Streptococcus agalactiae were identified in the tissue culture, and clindamycin and ceftriaxone were administered. Wound irrigation and serial sharp debridement using a curette were performed daily until there were no necrotic debris in the wound and it was covered by healthy granulation tissue.
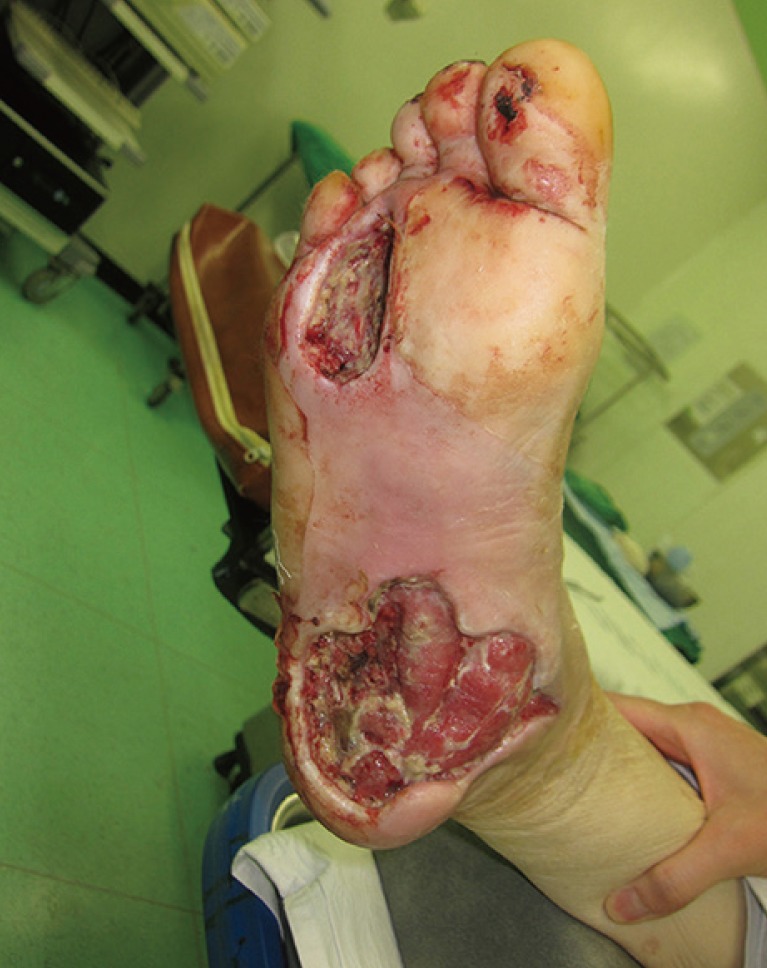
Preoperative photography. Soft-tissue defects in the forefoot (approximately 5 cm×4 cm) and hindfoot (approximately 9 cm×10 cm) are visible.
In the fourth week after the initial emergency room visit, soft-tissue coverage was planned using a thoracodorsal-axis chimeric flap. Before the operation, we performed an ankle-brachial index test and angiography to confirm the patency of the peripheral arteries.
After multi-detector computed tomography to locate a dominant perforator, a thoracodorsal artery perforator flap (approximately 8 cm×4 cm) was designed in the back. After the skin incision, the fasciocutaneous perforator flap was elevated, and its pedicle (descending branch of the thoracodorsal vessel) was completely skeletonized from the latissimus dorsi muscle. Further, a latissimus dorsi muscle flap (approximately 10 cm×10 cm) based on the transverse branch of the thoracodorsal vessel was dissected, and the chimeric flap was harvested (Fig. 2). The skin flap component was inset into the forefoot defect, and the muscle flap component was inset into the hindfoot defect. The thoracodorsal vessel was microanastomosed with the posterior tibial artery and its vena comitans. Finally, a split thickness skin graft was placed over the muscle flap.
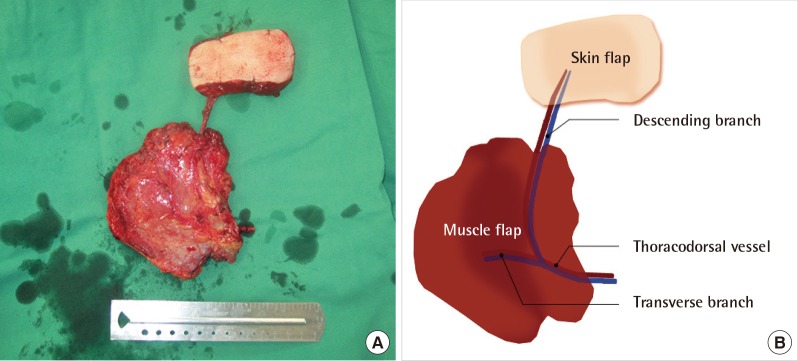
(A, B) Harvested thoracodorsal-axis chimeric flap. The skin flap component is nourished by the descending branch of the thoracodorsal vessel, and the muscle flap component is nourished by the transverse branch of the thoracodorsal vessel.
Four weeks after the procedure, complete weight bearing on the reconstructed area was allowed. During the 10 months of follow-up, no major complications occurred, including flap necrosis or wound disruption, and the patient did not feel discomfort upon wearing shoes or walking. A callus tended to form in the heel area, but this was alleviated after wearing specialized diabetic footwear. The contour of the reconstructed foot was satisfactory (Fig. 3).
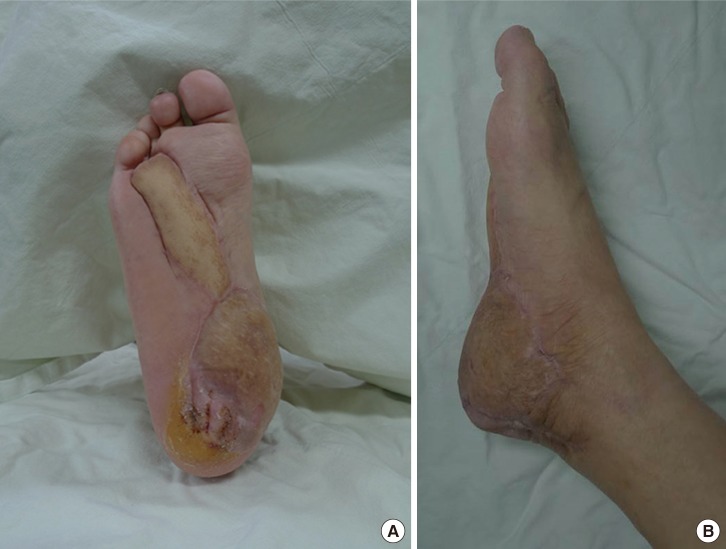
(A, B) Result at postoperative 10 months. Both flaps completely survived and withstood the daily stress of walking. The normal foot contour was also preserved.
Numerous techniques are used for reconstructing plantar soft-tissue defects, including skin grafts, local flaps, local intrinsic muscle flaps, and pedicled flaps. Although a skin graft can cover a large open wound, it cannot endure weight bearing. Surgery involving a local flap is simple to perform when adequate soft tissue can be obtained [12]. However, plantar skin and soft tissue are not redundant, limiting the feasibility of a local flap in the coverage of large wounds. A local muscle flap is also limited in size and location [2]. Of the pedicled flaps, a sural artery flap, which is frequently used for hindfoot defects, has high complication rates, particularly in patients with DM and arterial and venous insufficiency [2]. Therefore, free-flap surgery may be the most appropriate procedure to reconstruct DM-related soft-tissue defects in the large weight-bearing plantar area, as in the present case [1].
The chimeric flap, first introduced by Hallock, is basically a compound flap composed of multiple independent flaps that originate from different tissue types, each of which has its own vascular supply, with all pedicles converging to a common source vessel [34]. A separate skin flap, fasciocutaneous flap, muscle flap, or bone flap may be included in a single pedicle [4].
The chimeric flap has several advantages. First, it can achieve the same effect as a combination of 2 or 3 free flaps by expanding the surface of the flap without additional donor-site morbidity when an extensive soft-tissue defect needs covering [5]. Second, in cases where at least 2 free flaps are needed but the availability of a suitable recipient vessel is limited, such as in patients with head and neck cancer who previously received radiation therapy or patients with a DM-related occlusion of a major limb vessel in the foot, multiple flaps can be nourished using a single pedicle [3]. Third, the versatility of a chimeric flap offers a particular advantage in insetting, and the coverage of multiple or regional complex wounds is possible [3]. In our case, we could reconstruct the defects with a single chimeric flap while preserving the natural foot contour because each tissue component in the flap was completely separate and the pedicle of the skin flap component was quite long. We think that in this patient, the defect could be covered with one large fasciocutaneous flap after the removal of the plantar skin bridge between two defects. However, this method could destroy the normal plantar arch and result in a flatfoot deformity. Further, to preserve the plantar arch by using this method, the transition zone from hindfoot to forefoot should be narrowly designed; hence, the distal portion (forefoot) of the flap might have insufficient blood flow if the skin perforator is located in the proximal portion (hindfoot) of the flap.
This procedure is disadvantaged by the high level of difficulty and the risk of prolonged general anesthesia because of the long operation time. For these reasons, we recommend that surgeons try this technique after considerable experience with local or free perforator flap surgery and always have a second option in mind in case of flap failure. Further, a sufficient understanding of the wound characteristics, donor and recipient vessels, and design for insetting should precede the surgery. Lastly, the skin graft over the muscle flap is less durable than the fasciocutaneous flap and tends to form a callus. To prevent this complication, a perforator-based fasciocutaneous chimeric flap, which enables an independent inset of components, seems to be more ideal.
Notes
No potential conflict of interest relevant to this article was reported.