The Anconeus Muscle Free Flap: Clinical Application to Lesions on the Hand
Article information
Abstract
Background
It can be difficult to select an appropriate flap for various defects on the hand. Although defects of the hand usually must be covered with a skin flap, some defects require a flap with rich blood supply and adequate additive soft tissue volume. The authors present their experience with the anconeus muscle free flap in the reconstruction of various defects and the release of scar contractures of the hand.
Methods
Ten patients underwent reconstruction of the finger or release of the first web space using the anconeus muscle free flap from May 1998 to October 2013. Adequate bed preparations with thorough debridement or contracture release were performed. The entire anconeus muscle, located at the elbow superficially, was harvested, with the posterior recurrent interosseous artery as a pedicle. The defects were covered with a uniformly trimmed anconeus muscle free flap. Additional debulking of the flap and skin coverage using a split-thickness skin graft were performed 3 weeks after the first operation.
Results
The average flap size was 18.7 cm2 (range, 13.5–30 cm2). All flaps survived without significant complications. Vein grafts for overcoming a short pedicle were necessary in 4 cases.
Conclusions
The anconeus muscle free flap can be considered a reliable reconstructive option for small defects on the hand or contracture release of the web space, because it has relatively consistent anatomy, provides robust blood supply within the same operative field, and leads to no functional loss at the donor site.
INTRODUCTION
Optimal aesthetic and functional outcomes after the reconstruction of traumatic injuries of the hand cannot always be obtained even if the procedures are performed correctly. This is partly because the result of surgery depends on a range of factors, including the mechanism of injury, ischemic time, the degree of contamination, and soft tissue loss. Poor outcomes such as soft tissue defects, tendon or bone exposure, and scar contracture may occur, and the reconstructive options for these outcomes may be challenging. Serial reconstructive procedures should be carried out, and the transfer of well-vascularized tissue can be helpful for improving surgical results.
The muscle flap has not only been used to restore motor function [1,2], but also to cover various defects, including complex, complicated, contaminated, or chronic wounds [3,4]. Muscle flaps can be helpful for improving wound healing because they provide robust blood supply around the lesion. The use of a muscle flap, however, should be carefully considered when a defect requires a flap. Mathes and Nahai [5] proposed a classification of the vascular anatomy of muscle, and 5 patterns of muscle circulation were noted. Type I muscles usually have 1 dominant vascular pedicle. If a type I muscle can be used as a flap without significant donor morbidity, it can be considered a valuable option for some challenging wounds requiring vigorous blood supply.
The purpose of this study was to introduce a new muscle flap, the anconeus muscle flap, which provides abundant blood supply to various hand defects and is harvested in the same operative field under only brachial plexus block.
METHODS
A retrospective chart review was performed for all patients who underwent free anconeus muscle flap transfer for hand reconstruction between May 1998 and October 2013. Patients were included in the study if they had soft tissue defects with exposed tendon or bone, osteomyelitis, or web space contracture. No exclusion criteria for this method were applied. All patients provided written informed consent. Patients’ medical records were reviewed to obtain information regarding demographics, vessel characteristics, the anastomosis method, the total operative time, flap survival, associated complications, and whether any additional procedures were needed.
Operative technique
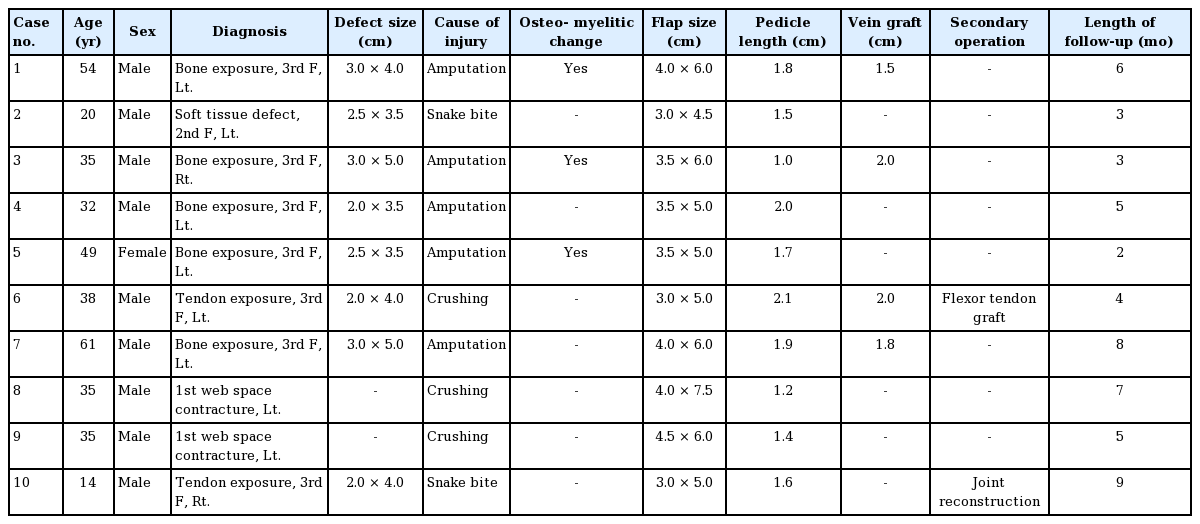
The anconeus free flaps were transferred under brachial plexus block. Patients were placed in the supine position with the elbow flexed at 90° over the patient’s torso. The intermuscular septum between the anconeus muscle and common extensor group was located with gentle palpation. After tracing the course of the recurrent posterior interosseous artery (RPIA) using portable Doppler imaging, a triangle was drawn on the skin over the anconeus muscle (Fig. 1). A line along the intermuscular septum connecting the lateral humeral epicondyle, the proximal ulna, and the tip of the olecranon made up the triangle. The tracing of the pedicle was sometimes difficult due to its location under the muscle masses.
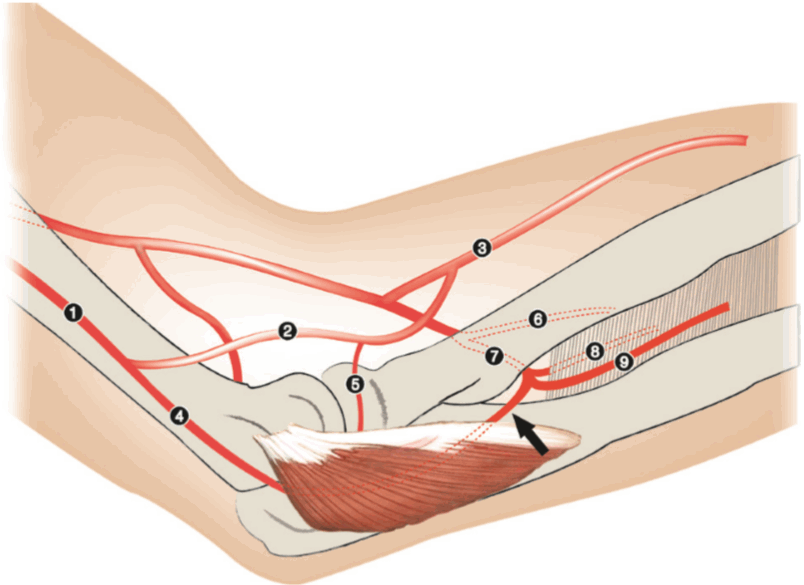
Schematic presentation of the anatomy
The recurrent posterior interosseous artery is shown at the deep surface of the anconeus muscle. 1, profunda brachii artery; 2, radial collateral artery; 3, radial artery; 4, middle collateral artery; 5, posterior branch of the radial collateral artery; 6, ulnar artery; 7, common interosseous artery; 8, anterior interosseous artery; 9, posterior interosseous artery; black arrow, recurrent posterior interosseous artery.
A longitudinal skin incision was made along the designed line from the lateral humeral epicondyle to the proximal ulna. The skin flap was retracted laterally to expose the muscle fascia, and the fascia was incised along the intermuscular septum between the extensor carpi ulnaris and the anconeus muscle. With the anconeus muscle gently retracted towards the operator, the flap pedicle was identified where it penetrated deeply into the intermuscular septum. With isolation of this pedicle, the anconeus muscle was elevated from the lateral humeral epicondyle. At the proximal margin of the muscle, the middle collateral artery (MCA) and posterior branch of the radial collateral artery (PBRCA) were identified and ligated. The distal portion of the anconeus muscle was further dissected, and the main pedicle, usually the RPIA, was carefully dissected. The anconeus muscle was elevated at the distal attachment site to the ulna bone, and the pedicle was ligated at the penetrating point of the intermuscular septum. After meticulous hemostasis and placement of a drain, the donor site was closed primarily. A mild compressive dressing was put in place on the donor site.
The harvested muscle flap was inset into the recipient site with several stitches. The artery and venae comitantes were anastomosed with recipient vessels under an operating microscope. If the recipient digital vascular bundle was not readily accessible, the wound was extended until a suitable portion was identified proximally. If the recipient vessels were too distant to be anastomosed without tension, a distal branch of the cephalic vein was harvested and used as an interposition graft. Intraoperatively, circulation was evaluated by releasing the tourniquet, and was confirmed by sanguineous color and bleeding from the flap margin. The flap and wound were covered with an immediate or delayed split-thickness skin graft.
A skin flap could not be used for monitoring flap viability because the anconeus muscle flap does not include a septocutaneous or musculocutaneous perforator branch to the skin. As such, the flap was monitored using portable Doppler imaging. No anticoagulants were used.
RESULTS
The review identified 1 female patient and 9 male patients, with a mean age of 37.3 years (range, 14–61 years). Right hand injuries were observed in 2 patients, and left hand injuries were present in 8. All the patients were right-handed.
Anconeus free flaps were used to resurface the index fingers (n=4), long fingers (n=4), and the first web space (n=2). The injury mechanisms were amputation (n=5), crushing (n=3), and snake bite (n=2). The indications for reconstruction were tendon or bone exposure (n=7), a skin and soft tissue defect (n=1), and web space contracture (n=2). Signs and symptoms of osteomyelitis were observed in 3 of the 7 patients with tendon or bone exposure (Table 1).
The mean length of flap pedicle was 1.6 cm (range, 1.0–2.1 cm). The mean arterial diameter was approximately 1 mm, with the respective veins having similar or slightly wider diameters. The flap vessels were anastomosed to the digital artery (n=8) or to the superficial branch of the radial artery (n=2), the latter of which required end-to-side anastomoses. Interposition vein grafts were required in 4 cases. The mean operative time was 179 minutes (range, 120–220 minutes). The flaps survived well with successful skin graft take in all cases, without significant complications.
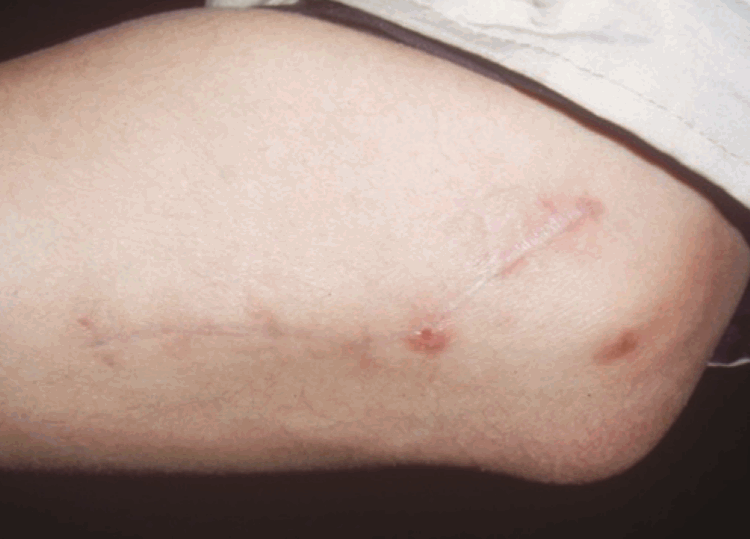
With regard to the donor site, all the patients were able to move their elbow and forearm through the full range of motion and did not experience any instability or weakness of the elbow in extension. The follow-up period ranged from 2 to 9 months (mean, 5.2 months).
Case 1 (Figs. 2–8)
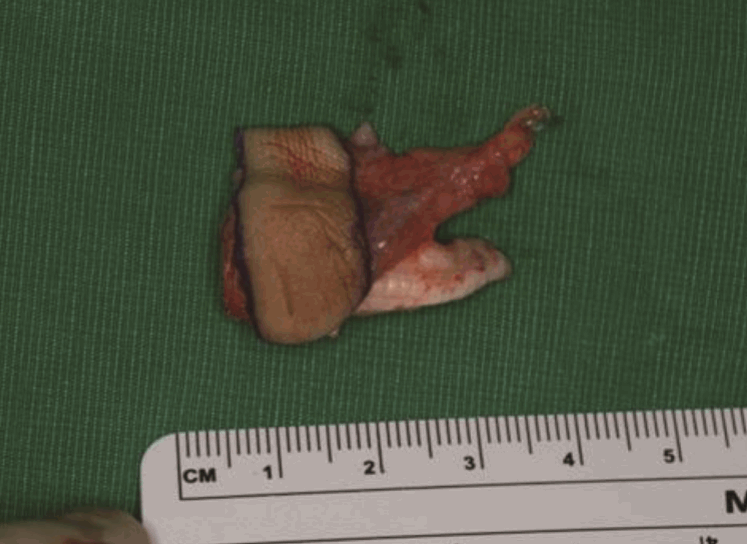
Case 1: a 54-year-old male patient
A 54-year-old male patient sustained an amputation with avulsion of the left middle finger and underwent replantation. After 3 weeks, the defect showed bony exposure with osteomyelitis on the middle phalanx and fingertip.
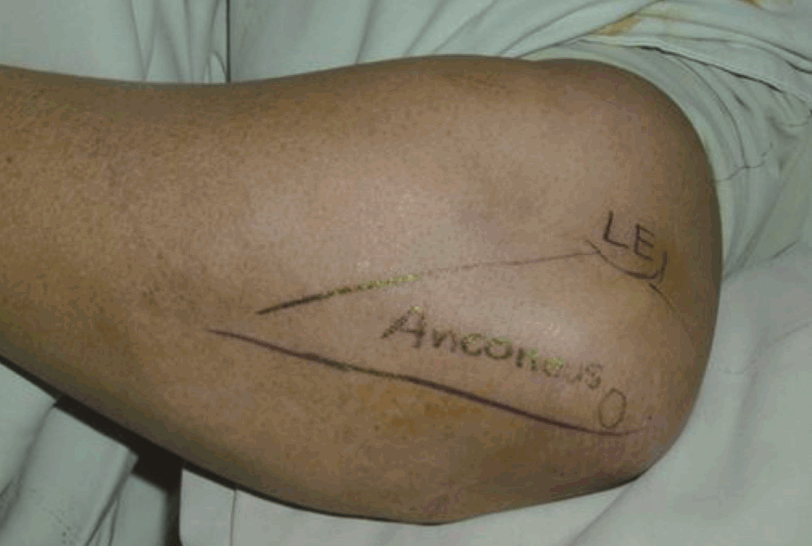
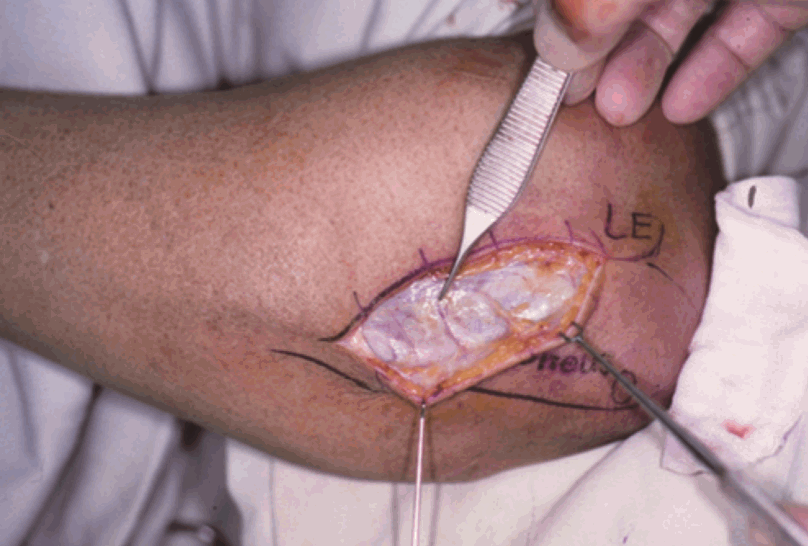
Surface anatomy of the anconeus muscle
After skin incision, suprafascial plane dissection was performed. LE, lateral epicondyle of the humerus; O, olecranon.
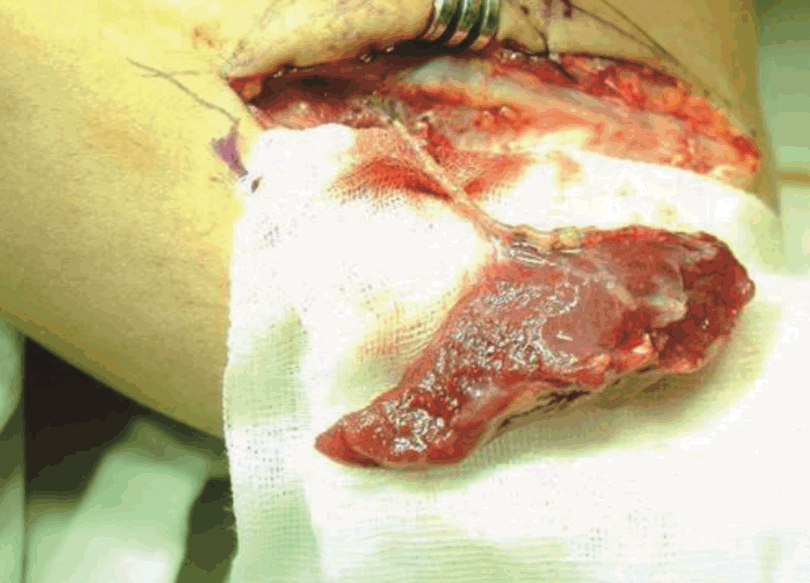
View during superficial dissection
Adson forceps indicate the intermuscular septum between the anconeus muscle and common extensor group. LE, lateral epicondyle of the humerus; O, olecranon.
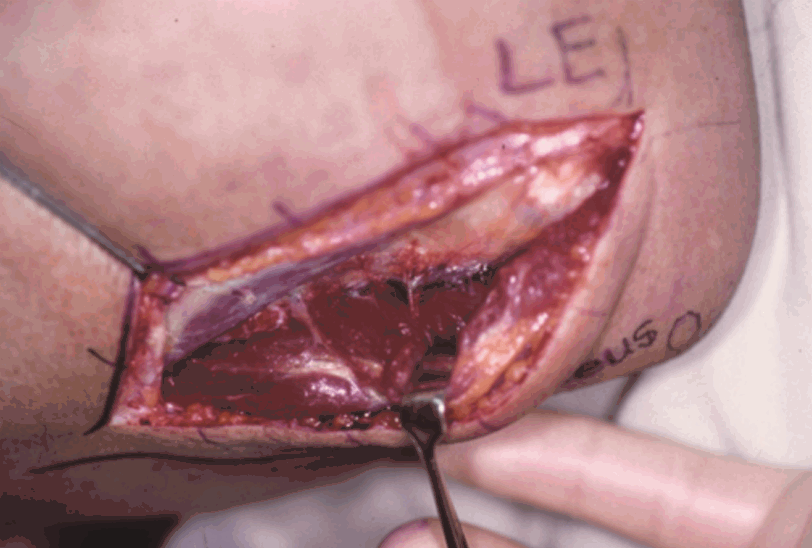
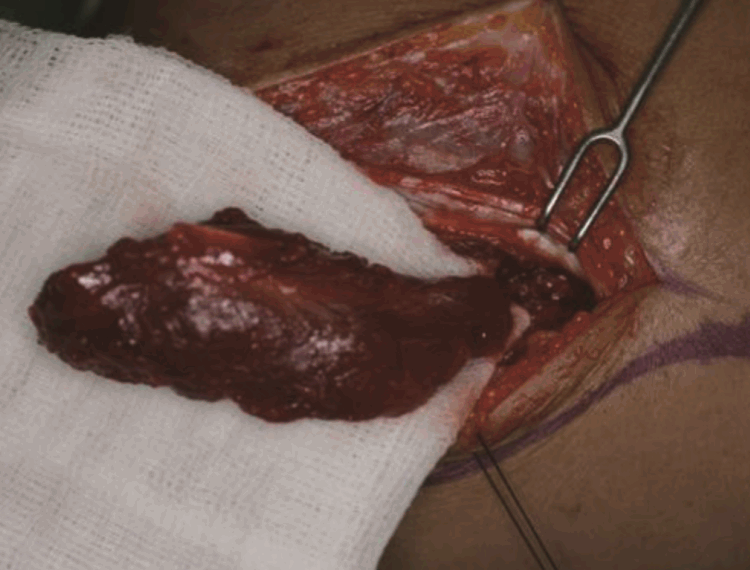
View during deep dissection
The recurrent posterior interosseous artery is shown on the deep surface of the anconeus muscle. LE, lateral epicondyle of the humerus; O, olecranon.
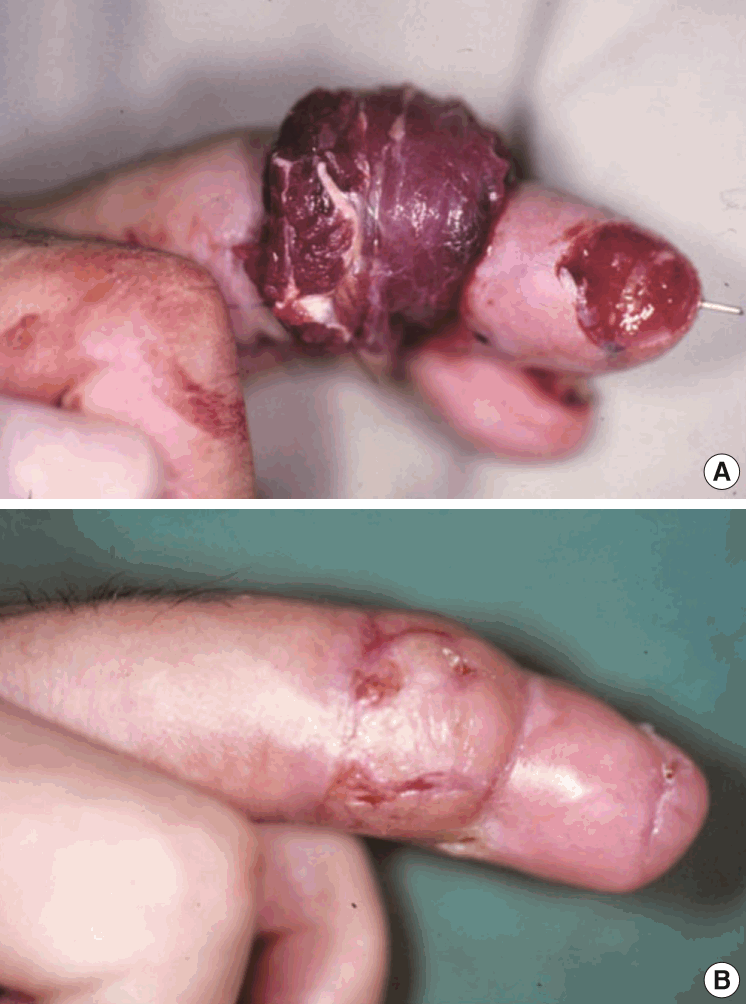
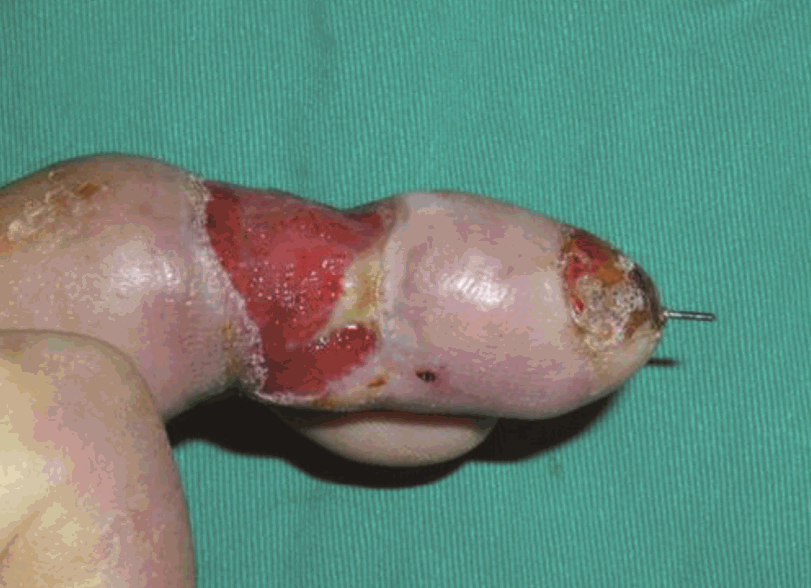
Postoperative photographs
(A) Immediate postoperative view. (B) Postoperative view after 2 months. Coverage of a defect site with an anconeus muscle free flap. Debridement was performed on the fingertip. The flap survived without complication.
This 54-year-male patient was injured while using a lathe and presented with an avulsion-type amputation of the left middle finger. The amputation was at a level distal to the proximal interphalangeal joint level and was replanted. Three weeks later, the radial side of the middle phalanx became necrotic. Serial debridement of this necrotic tissue left a 3×4-cm defect with exposed bone. Despite meticulous wound care, the wound began to drain purulent discharge due to osteomyelitis of the middle phalanx. To bring the osteomyelitis under control, the defect was covered with an anconeus muscle free flap using the radial digital artery and the volar vein as a recipient vein. Due to infection and scarring, a 1-cm portion of the recipient artery was excised, and a vein graft was interposed between the healthy portion. No further evidence of osteomyelitis was found.
Case 10 (Figs. 9–15)
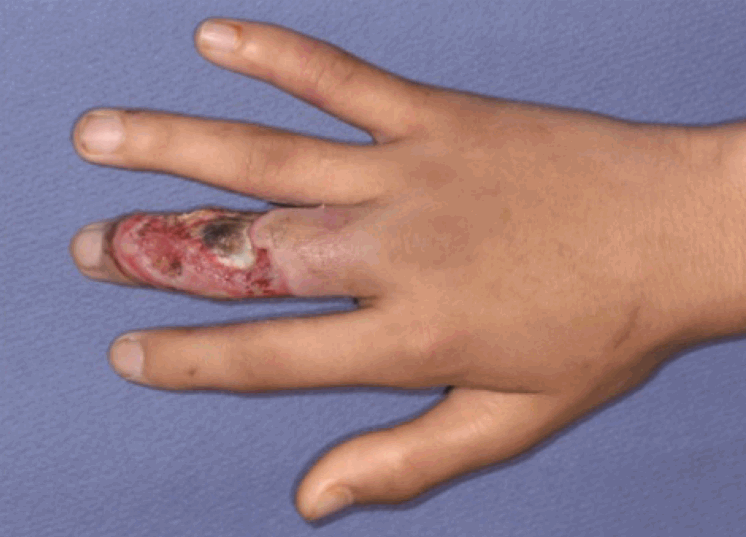
Case 10: a 14-year-old male patient
A 14-year-old male patient with a skin and soft tissue defect on the right long finger.
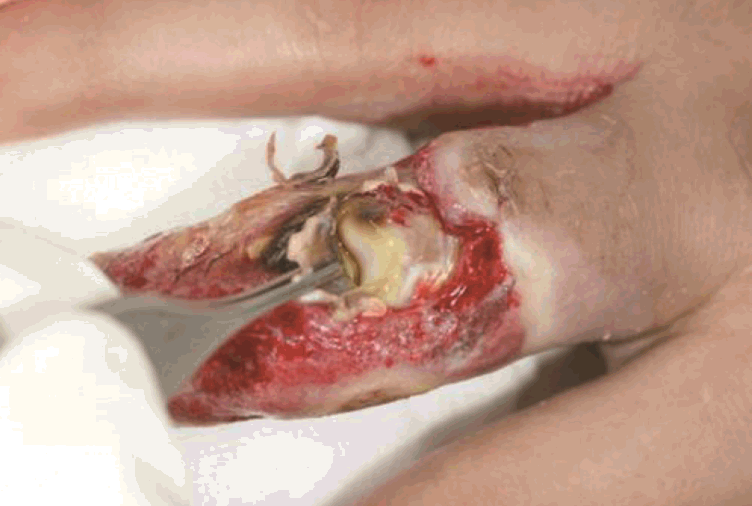
View during debridement
The proximal interphalangeal joint, bones, and extensor tendon were exposed.
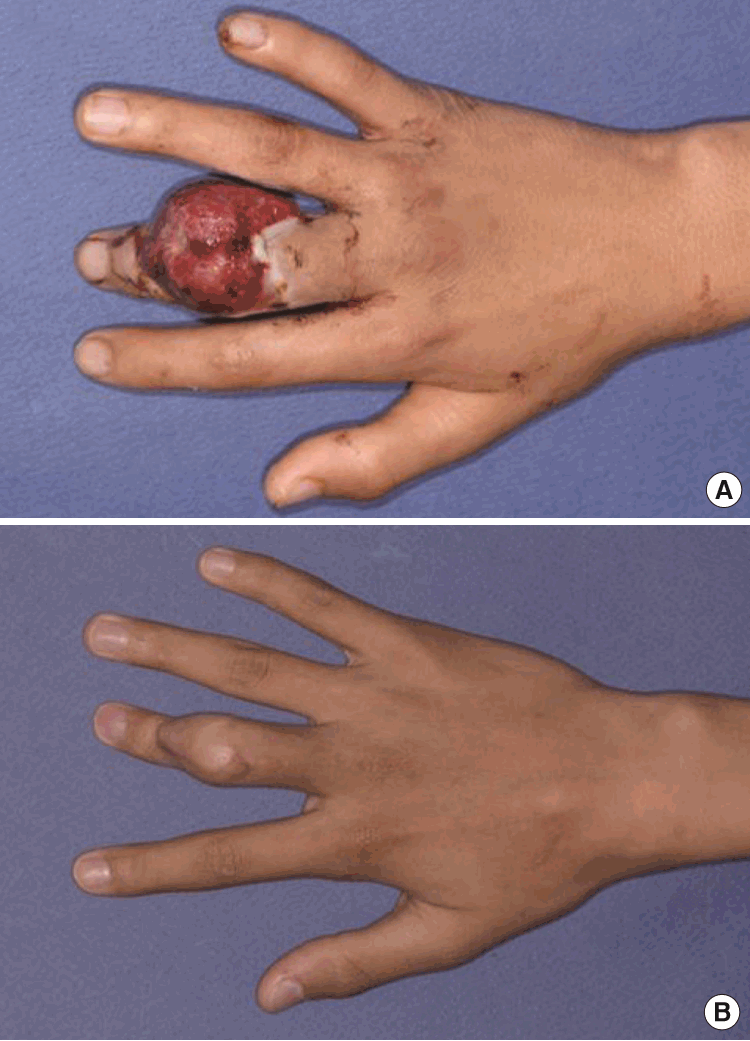
Postoperative photographs
(A) Postoperative view after 3 weeks. (B) Postoperative view after 9 months. Three weeks after the first operation, tangential excision for debulking of the flap and a split-thickness skin graft were performed. Limited motion at the proximal interphalangeal joint was observed.
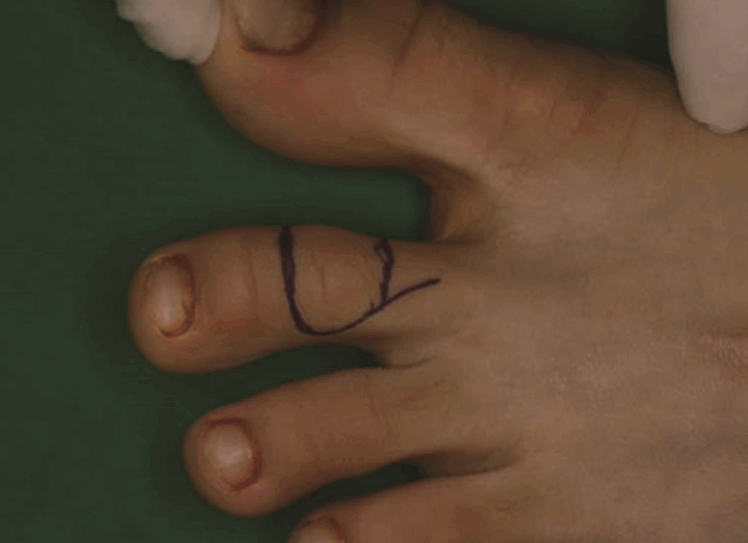
Design of the second toe joint free flap
The reconstruction of the proximal interphalangeal joint of the right long finger was planned with the proximal interphalangeal joint of the left second toe as a donor site.
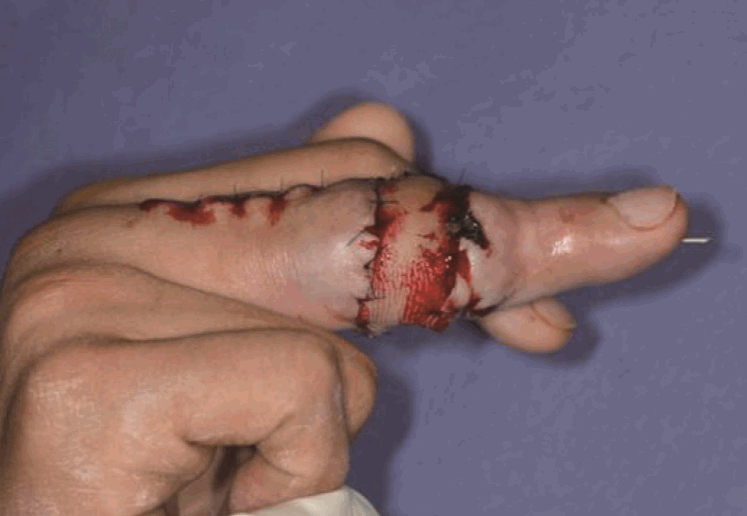
Postoperative view after 1 week
The contracture of the proximal interphalangeal joint was released and straightened.
The right long finger of this 14-year-old male patient was bitten by a venomous snake. Despite close observation and proper wound care for 3 weeks, the skin and soft tissue over the long finger underwent full-thickness necrosis and the patient was transferred to our institution for further management.
An anconeus muscle free flap was selected for reconstruction not only to cover the 2×4-cm defect exposing the extensor tendon but to control the persistent soft tissue infection. Under brachial plexus block, adequate debridement was done and followed by coverage with an anconeus muscle free flap. The muscle flap survived without significant complications and a split-thickness skin graft was performed 3 weeks postoperatively.
Defect coverage and infection control were successful, but the patient complained of stiffness of the proximal interphalangeal joint. The patient and his parents were concerned for the stunted growth of the finger because the injury was in his dominant hand. To address the concern of finger length, a toe-to-finger joint transfer was planned and executed 9 months after the anconeus muscle transfer. This vascularized joint was taken from the left second toe and replaced the proximal phalangeal joint of the involved finger.
No significant complications occurred at the flap site or at the donor site, and bony healing is continuing.
DISCUSSION
The anconeus muscle belongs to a group of posterior superficial muscles in the forearm. This small, pyramid-shaped muscle assists the triceps in extension of the elbow and pronation and supination of the forearm. It stabilizes the elbow joint through its motion, and loss of this muscle is associated with minimal functional deficits [6,7]. The anconeus muscle is usually nourished by 3 arteries: the MCA, the PBRCA, and the RPIA. The last of these has the largest mean diameter (1.1±0.4 mm or 1.9±0.2 mm) and has a consistent anatomical pattern. The artery has been reported to have a mean length of 31.3±6.9 mm, which is adequate for microsurgical anastomosis [8,9]. Several publications have described the vascular anatomy of the anconeus muscle and the use of this muscle as a local flap [8,10]. Most recently, this muscle was considered for use as a functional free flap for thenar reconstruction, but this possibility was explored in a cadaveric study without any clinical demonstrations [9].
It is well known that complications following trauma to the tubular bones of the hand are uncommon [11,12]. This can partially be explained by the extensive blood supply of the phalangeal and metacarpal bones [13,14]. Conversely, any kind of trauma or insult to the hand that threatens blood supply can cause a range of wound complications, and such complications should be addressed by improving blood circulation to the lesion.
Musculocutaneous flaps were introduced first by Ger [15] and again by Orticochea [16] a decade later. Although several authors have reported the use of muscle flaps for various reconstructive purposes [17,18], earlier studies did not provide strong support for the use of a muscle flap as a reliable source of blood supply for a defect or an infected dead space. However, others have discovered that muscle flaps are effective in the treatment of complex and complicated wounds, including the management of intractable osteomyelitis [19]. Several experimental studies have demonstrated that muscle flap coverage could significantly increase blood flow to devascularized bone segments in comparison with skin coverage only. Moreover, the use of a muscle flap could promote the early return of strength across an osteotomy [4,20]. In general, muscle flaps provide more vascularity to a defect than do fasciocutaneous flaps [21,22]. These findings imply that muscle flaps could be a reliable solution for chronic soft tissue infections, coverage of exposed bone, or intractable osteomyelitis [23,24].
As with all forms of reconstruction using autologous tissue, however, serious consideration should be given to donor morbidity at the location where the muscle harvest is planned, because loss of motor function and weakness of structural support have frequently been reported [25]. Although the anconeus muscle does have a relatively uncommon mechanical function, it is relatively small and causes a minimal functional deficit after flap harvest. Hence, it can be a potent treatment option for providing robust blood supply to a small wound with relatively poor circulation.
As a result of its location in the forearm, the anconeus muscle flap can be harvested within the same operative field as the injured hand, which allows the operation to proceed with a single brachial plexus block. This simplification of the operative field and anesthesia shortens the operative time and means that a positional change is not required. The elbow region is not commonly used as a donor site, and its blood vessels are usually too short to serve as vascular pedicles. However, the operative field is neither wide nor deep; therefore, the anconeus muscle free flap does not have a steep learning curve.
Using anconeus free flaps, the authors treated 5 defects with bone exposure (of which 3 were associated with osteomyelitis), 3 defects with soft tissue or tendon exposure, and 2 cases of web space contracture. Although the muscle flap was not the only factor that influenced the results, the vascularity recovered or augmented by the flap was very helpful in obtaining satisfactory outcomes. The anconeus muscle free flap fulfilled this role satisfactorily without significant donor morbidity.
Notes
No potential conflict of interest relevant to this article was reported.
Notes
PATIENT CONSENT
The patient provided written informed consent for the publication and the use of their images.