The Effect of Hyperbaric Oxygen Therapy on a Large Composite Graft in an Ear Amputated by a Human Bite
Article information
Traumatic amputation of the ear helix is relatively a rare occurrence. Various reconstruction techniques have been reported for this condition [1]. We present the case of a 41-year-old female patient who sustained a large traumatic amputation of the ear helix due to a human bite. Composite graft surgery was performed with adjunctive hyperbaric oxygen therapy (HBOT). The graft successfully survived, with favorable cosmetic results.
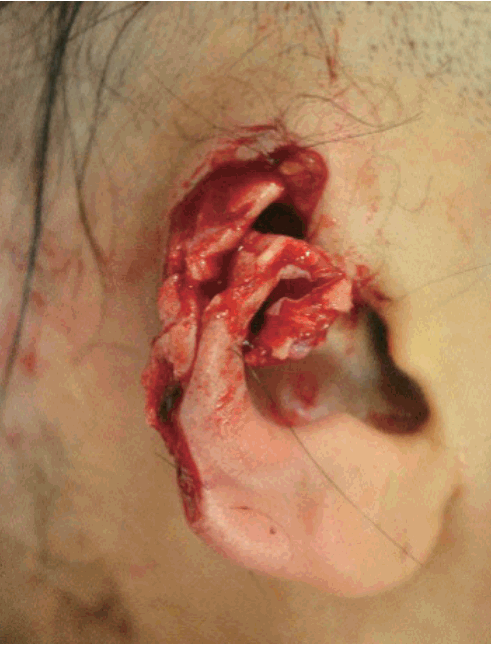
A 41-year-old female patient presented to our emergency center with a partially amputated right ear helix (Fig. 1). Due to a human bite injury, the helix was irregularly amputated, with the cartilage exposed. The amputated ear helix measured 4×3 cm. Reconstructive surgery was performed immediately under general anesthesia. The vascular status of the amputated ear was evaluated; however, due to the nature of its avulsion, there were no suitable arterial vessels. After debridement of the wound margin and rugged cartilage, a composite graft of the amputated helix was performed. Adjunctive HBOT was applied to enhance the reperfusion of the wound and for infection control over the course of 7 days. A piece of plastic bag was placed with an oxygen tube connected around the wound. We sealed the plastic bag around the wound to prevent air leakage (Fig. 2). Many holes were made by needles in the sealed plastic bag around the wound, so that the continuously supplied air escaped from the plastic bag. The flow rate was 5–6 L per minute for 24 hours.
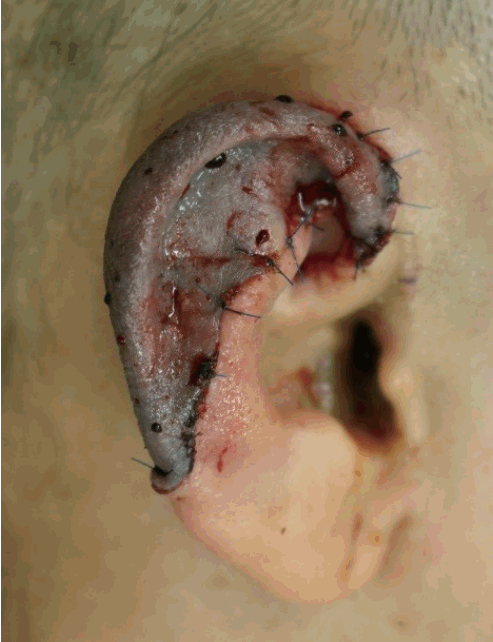
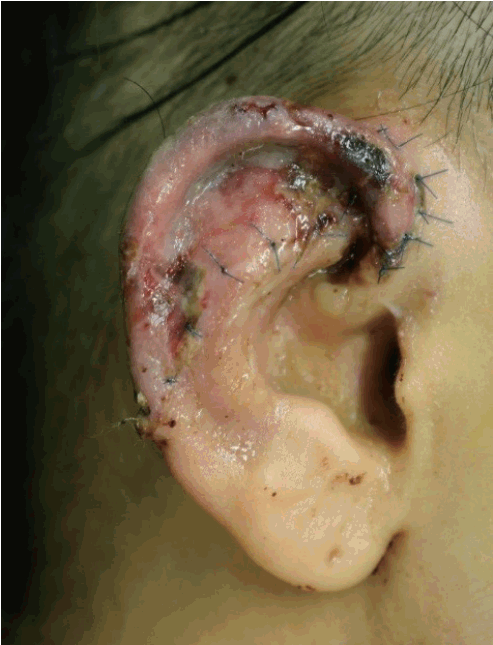
Initially, our patient was pale, without any reaction to the pinprick test. On postoperative day 2, the color of the amputee in the affected area changed to pink and purple. Mild congestion was noted, with a reaction to the pinprick test (Fig. 3). The capillary refill time was determined to be 1 second. At first, inflammatory signs such as redness, swelling, tenderness, and heating sensation were observed in the wound. By 5 days after the operation, the signs of inflammation had decreased, as seen on the physical examination, and they disappeared by 7 days after the operation. After postoperative day 10, the graft survived nearly completely, with minimal skin necrosis (size, 0.3×0.3 cm) (Fig. 4). Two weeks after surgery, the patient was discharged without significant complications.
Ear amputation is not a common event, and it remains challenging to reconstruct. Microvascular replantation is the best method to treat ear amputations. In most cases, however, the severely injured vessels and small vessels make microanastomosis especially challenging. Moreover, a high risk of infection may hinder the chances of the amputee’s survival [1]. For patients without a suitable vessel for microanastomosis, non-microsurgical methods are suggested, such as a composite graft, retroauricular pocket, or a temporoparietal fascia flap. A few reports of large composite grafts to the ear have been published. The survival rate of composite grafts is lower than that of replantation with microvascular anastomosis. The vessel status of amputees who have sustained a human bite injury is often not suitable for anastomosis. Moreover, the risk of wound infection is often increased, as in our case. A variety of adjunctive treatments, such as the administration of heparin, prostaglandin, leeches, and HBOT, have been introduced for patients who undergo a composite graft. However, these treatment options have not yet been established.
In our case, after a composite graft, we performed HBOT. This approach has 2 advantages with respect to wound healing. First, it is known to promote healing by increasing reperfusion [2]. In cases of a composite graft without microvascular anastomosis, it is crucial for adequate blood circulation to be supplied during wound healing. This increases angiogenesis and stimulates fibroblast proliferation [2]. Thus, the survival of compromised skin grafts and flaps can be increased by oxygen therapy [3]. Oxygen therapy has also been proven to have a bactericidal effect [4]. In cases of human bite injury, there is a higher risk of infection than in injuries due to other causes because of the vast array of bacterial species in the mouth [5].
A few case reports have described employing HBOT as an adjunctive method for composite grafts. However, in order to implement HBOT, equipment such as a high-pressure oxygen hood or a chamber is necessary. Such equipment is not available in many hospitals. We performed adjunctive HBOT by connecting oxygen to a plastic bag around the wound at the patient’s bedside. In our case, HBOT was employed and was effective for improving composite graft survival. Compared to conventional methods of administration using a chamber or a hood, this method can be more easily applied to patients if it is possible to supply pure oxygen at the bedside. Therefore, if a chamber or a hood for HBOT is not available, our method can be used as a treatment strategy.
Notes
No potential conflict of interest relevant to this article was reported.
Notes
PATIENT CONSENT
The patient provided written informed consent for the publication and the use of their images.