A decision-making method for breast augmentation based on 25 years of practice
Article information
Introduction
Breast augmentation is the most commonly performed procedure in the field of aesthetic plastic surgery [1,2]. Women considering breast enhancement are mostly interested in shape improvement: 29% wish to improve the size or volume of their breasts, 15% wish to improve the general shape, 29% wish to return to a previous shape (pre-breastfeeding), 32% would like to address sagging of their breasts, and 8% wish to improve symmetry.
Accurate preoperative planning is crucial for obtaining the best outcomes and for reducing the re-intervention rate. The entire decisionmaking process in breast augmentation was initially determined exclusively by the patient’s wishes and the surgeon’s preferences, making the choice of implant size, type of implant, implant position, and type of incision an arbitrary decision. This led to high re-intervention rates due to patients’ dissatisfaction with the implant size and other postoperative complications [3-5].
Many techniques aiming to refine the preoperative decision-making process for breast augmentation have been developed in the last 10 years, leading to a significant reduction of the re-operation rates [6-8]. Lower re-intervention rates are associated with the application of tissue-based planning methods and decision-making systems matching implants to patients’ tissues and breast dimensions [9-12]. Herein, we present our planning method, which is derived from more than 25 years of experience in aesthetic breast surgery and involves matching the characteristics of patients’ tissues with their wishes. We schematized our planning method in an easy-to-use flow diagram to help the decision-making process in breast augmentation.
Our planning method for breast augmentation
We firmly believe that a scientific and rigorous approach towards breast augmentation is mandatory in order to obtain good outcomes, long-lasting results, low complication and re-intervention rates, and high patient satisfaction levels. A rigorous approach starts with conducting an accurate first consultation, listening to the patient’s wishes, analyzing the characteristics of skin and soft tissues, evaluating the size of the chest wall and the breast, and assessing the breast shape, always remembering that if you fail to plan, you plan to fail.
After accurate planning and shared decision-making, a properly performed operation with a complete knowledge of the devices used, with a correct and standardized follow-up, are the next factors contributing to the best and longest-lasting results in breast augmentation.
We always have to balance the wishes of the patient with her tissue characteristics, identifying potential mismatches between the desired result and soft tissue characteristics. When the patient’s wish is recognized to be not achievable, further consultation and patient education are mandatory. External sizers are a very useful tool to enhance patient understanding of the achievable results during the consultation. The more clearly the patient’s expectations are defined and the better her specific wishes are communicated, the more likely our goals can be achieved.
We developed a planning method to guide the decision-making process in breast augmentation based on the characteristics of the skin and soft tissue, the breast and chest wall size, the breast shape, and the patient’s wishes. When planning a breast augmentation, the surgeon should assess the implant size, implant type, implant pocket position, and incision location, and each decision has a strong impact on the final outcomes. We must pursue evidence-based surgery, and to achieve predictable outcomes with low re-operation rates, we must make decisions based on objective data.
Several dimensional systems have been developed to assess implant size preoperatively before breast augmentation. One of the most commonly used systems is the BioDimensional System licensed by Inamed Corporation in 1994 [13], which later evolved into the TEPID system, a planning method that prioritizes long-term outcomes and minimizes the re-intervention rate [14]. We firmly believe that methods for assessing implant size should reconcile the patient’s wishes with her tissue characteristics, helping women understand the real possibilities of augmentation in light of their tissue characteristics and the limits of the achievable outcomes, based on objective, quantifiable measurements.
The final breast shape will depend on the characteristics of the coverage tissue (breast skin, glandular parenchyma, and fat) and implants. After an objective assessment of a specific patient’s parameters (chest wall width; base width of the existing breast; nipple-to-inframammary fold (IMF) distance under maximal stretch; medial, lateral, superior, and central pinch thickness of the existing tissues; cleavage; sternal notch to nipple distance), the surgeon can choose the best width, height, and projection of the implant (Fig. 1).
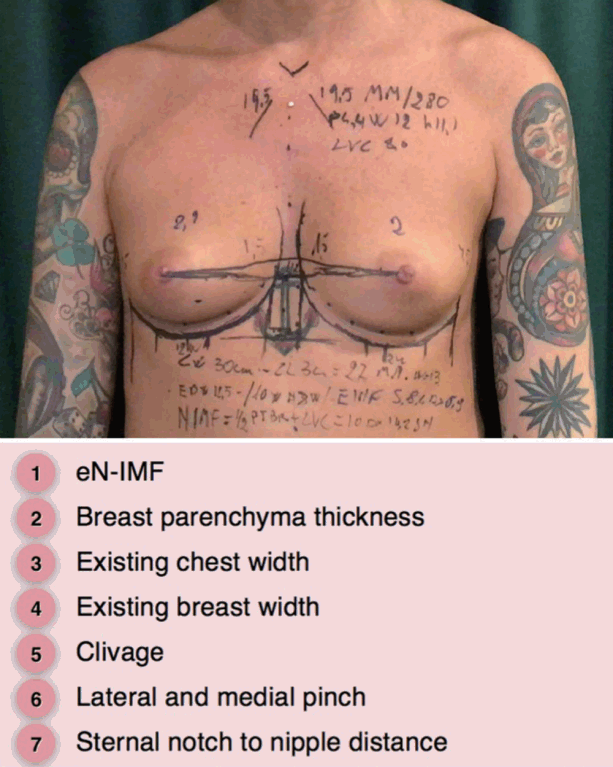
Preoperative planning of breast augmentation: key measurements. eN-IMF, existing nipple inframammary fold.
The dimensions determine the volume, not vice-versa. The surgeon must assess the breast volume, which is classified as very small, small, or medium. For medium-sized breasts, ptosis must also be assessed. Low or moderate ptosis can be solved exclusively with the correct use of the best anatomical implant (Allergan Style 510, Allergan, Irvine, CA, USA), while moderate to high ptosis requires adjunctive procedures (i.e., mastopexy), always trying to minimize implant contamination (Figs. 2, 3). The surgeon must also consider the patient’s wishes. Women requesting a full upper pole are offered an Extra-Projected Style 410 Cohesive Gel Implant (Allergan) (Fig. 4). Women wishing a more sloping upper pole are offered an Extra-Projected Style 510 Dual Gel Implant (Allergan) (Figs. 5, 6). If a soft breast is desired, the surgeon must consider a Low, Medium or Full Projected Style 410 Soft Touch Gel Implant (Allergan) (Fig. 7).
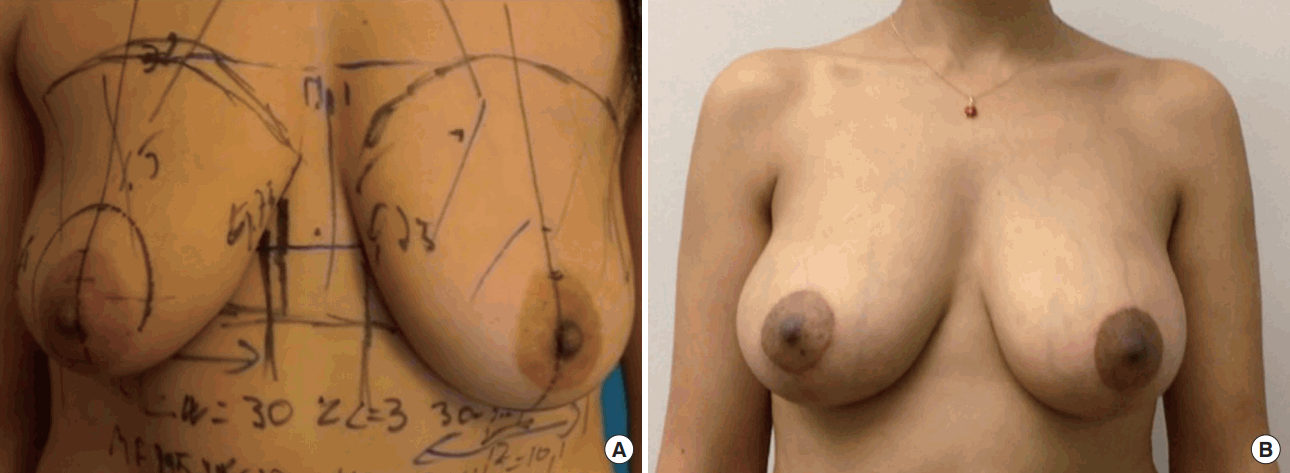
Wise-pattern incision using Allergan Style 410 MF Implant (width, 12 cm). Preoperative view (A). Postoperative follow-up at 1 year (B).
MF, medium (height)-full (projection).
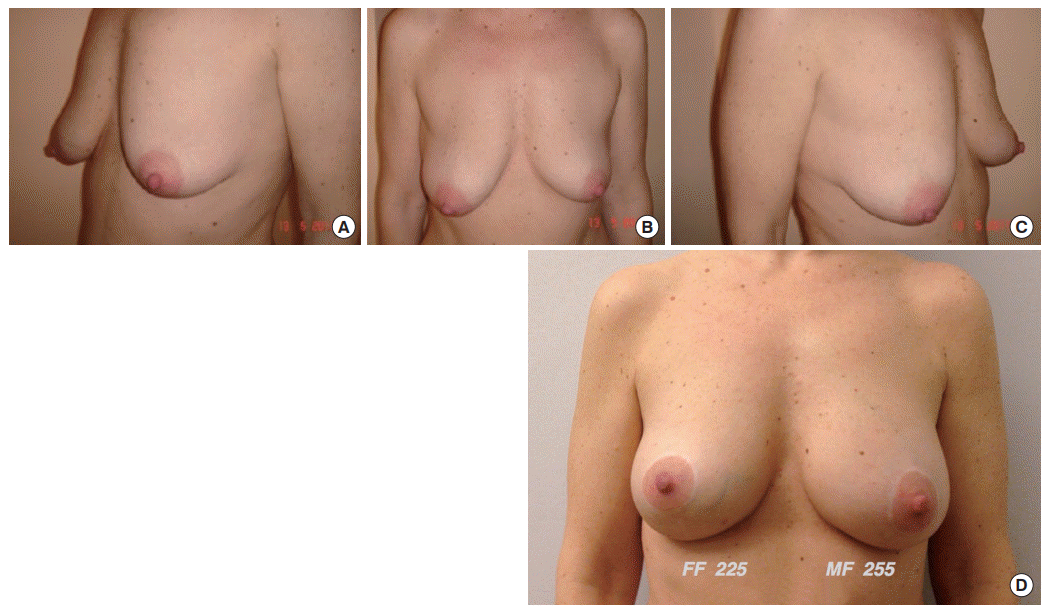
Allergan Style 410 FF Implant (volume, 225 mL) on the right side and 410 MF Implant (volume, 255 mL) on the left side. (A, B, C) Preoperative view. (D) Postoperative follow-up at 5 years. FF, full (height)-full (projection); MF, medium (height)-full (projection).
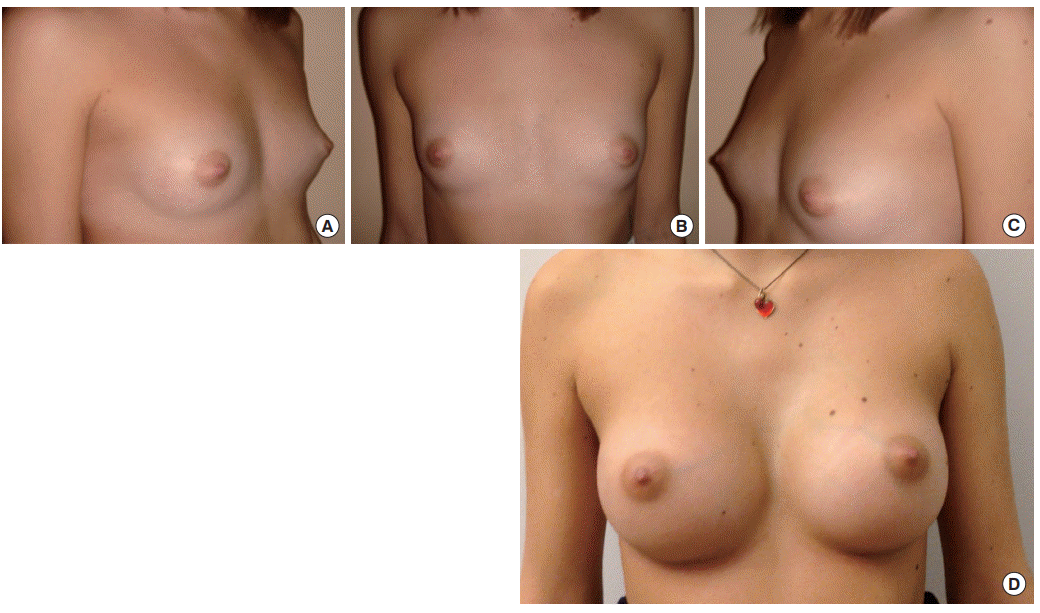
Allergan Style 410 MX Implant (width, 12 cm; height, 11.1 cm; volume, 325 mL). (A, B, C) Preoperative view. (D) Postoperative follow-up at 4 years. MX, medium (height)-extra (projection).
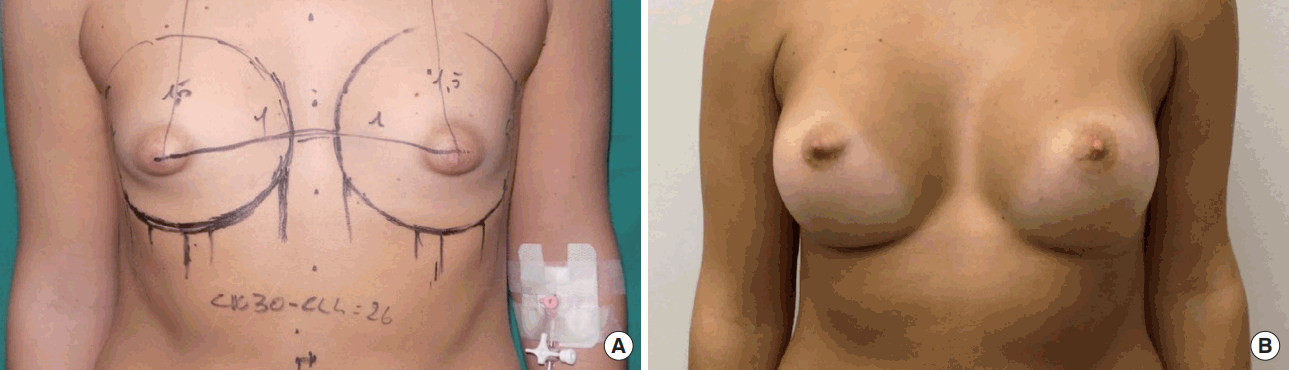
Allergan Style 510 Implant (width, 11.5 cm; volume, 245 mL). Preoperative view (A). Postoperative follow-up at 1 year (B).
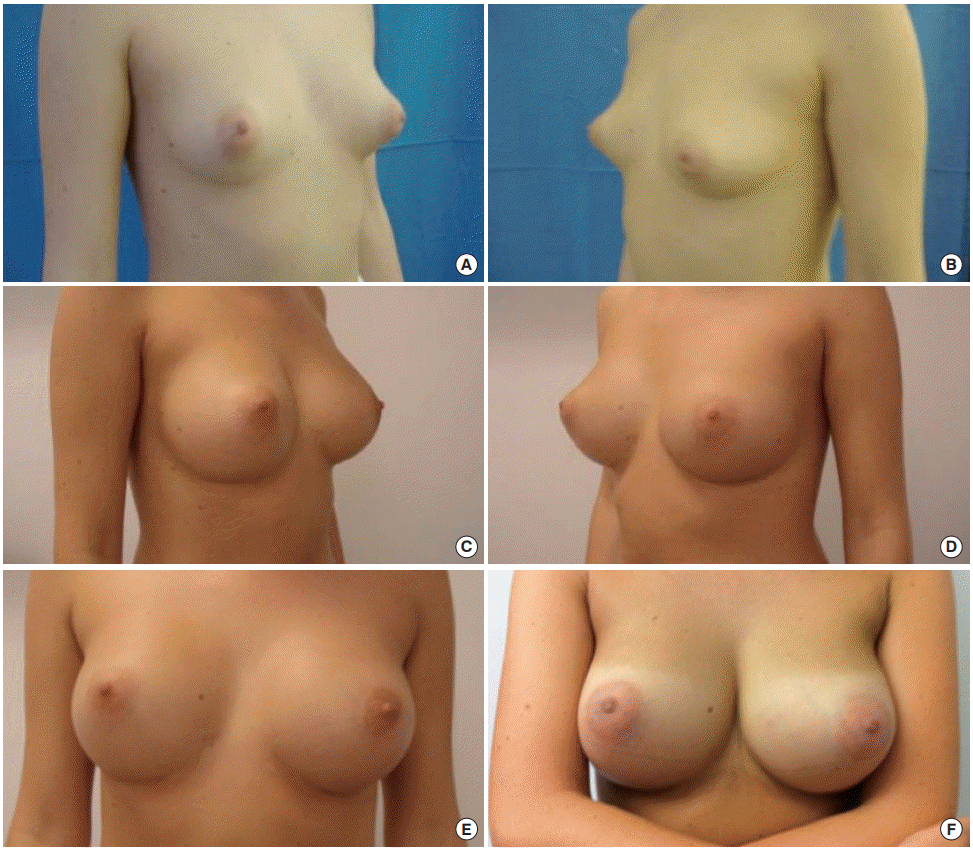
Allergan Style 510 Dual Gel Implants (width, 12 cm; height, 11.1 cm; volume, 290 mL). Preoperative view (A, B). One-year (C, D), 3-year (E), and 6-year (F) follow-up.

Allergan Style 410 FF Soft Touch implant (width, 11.5 cm; height, 12 cm; volume, 290 mL). (A, B, C) Preoperative view. (D) Postoperative follow-up at 6 years. FF, full (height)-full (projection).
Although good outcomes can be obtained with both round and anatomical implants in women with good breast tissue coverage, we prefer anatomical implants. They help to enhance the cosmetic results, allowing long-lasting results, and remain mandatory in challenging situations, when correcting congenital malformations, and when considering breast augmentation in very thin patients or patients with low/moderate breast ptosis [15,16].
The surgeon then assesses the skin and soft tissue characteristics, in terms of the medial, lateral, superior and central pinch thickness of the soft tissue. If the upper pole pinch thickness is less than 2 cm (medium/poor soft tissue), the surgeon should consider a dualplane technique to ensure good tissue coverage [17]. In case of very good soft tissue (upper pole pinch thickness of more than 2 cm), the surgeon should choose a sub-fascial breast augmentation. The use of a sub-fascial breast augmentation with a Style 510 Dual Gel Implant (Allergan) can also be considered in young women with a pinch thickness less than 2 cm, with the possibility of a re-intervention after 20–30 years, when they could undergo a dual-plane procedure with a Style 410 Cohesive Gel Implant (Allergan, Irvine, CA, USA).
Our preference for the incision location is at the IMF, in order to minimize implant contamination. However, the incision location should be chosen based on the patient’s wishes, in light of the surgical skills needed to reduce tissue trauma and the accompanying trade-offs.
When considering an incision at the IMF, estimating the level of the new IMF appears mandatory. Several methods have been described in order to define the level of the new IMF, such as the ICE principle [18] or the method reported by Tebbetts [14] along with the TEPID system. Other authors prefer to calculate the position of the new IMF by adding half of the parenchymal thickness to the implant’s lower ventral curvature. This new IMF calculation method has been validated with Allergan implants, and we currently do not know whether it can be extended to other types of implants. Our preference for new IMF positioning derives from an extension of Tebbetts’ method: the new IMF position is calculated by adding half of the width of the implant to a measure derived from the patient’s tissue thickness: if a small amount of tissue is present (pinch thickness less than 1 cm), we add 1 cm, if a moderate amount of tissue is present (from 1 to 2 cm), we add 0.5 cm, and if a satisfactory amount of tissue is present (more than 2 cm), no further additions are considered.
Our decision-making process for breast augmentation is summarized in the breast augmentation flow diagram (Fig. 8).
We firmly believe that the best outcomes in breast augmentation can only be achieved through standardized preoperative planning of the surgical procedure, a complete knowledge of the available devices, the application of an impeccable surgical technique, and appropriately scheduled follow-up.
The preoperative planning should reflect a balance between the patient’s tissue characteristics and the patient’s wishes. Quantifiable, objective parameters should drive decisions about implant choice and implant pocket position, but the patient’s desires can further define the final outcome if the surgeon has a clear sense of the entire “armamentarium” that is available for “scientific” breast augmentation.
Pinch thickness guides decisions about implant coverage and pocket location, while chest wall width, breast base width, nippleto-IMF distance, and skin stretch drive implant volume assessment, and quantitative techniques are used to define the new IMF position. Objective measurements help obtain long-lasting results that fulfill women’s desires, significantly reducing the re-intervention rate.
When considering a specific volume, implant width is the most important parameter, but the surgeon must take into account the height of the implant as well, depending on the characteristics of the overlying tissues, implant filler characteristics, and implant shell-filler interactions. When using non-form-stable implants, the height of the device is difficult to measure accurately, so implant width and projection remain the most significant parameters.
Accurate measurements are necessary, but so is impeccable surgical technique and standardized follow-up. Our recommended followup starts at 1 week, when the drapes are changed, and then paper tape is maintained on the surgical scars for 2 months, vigorous muscular exercise is avoided for 3 months, and post-surgical bras are worn day and night for 2 months and then only at night for 1 more month. Clinical evaluations are performed in the first, second, and sixth months after surgery and then yearly, together with breast imaging.
Our breast augmentation experiences
From September 2000 to December 2014, we performed 650 breast augmentations, including 208 (32%) sub-glandular and 442 (68%) dual-plane procedures, using Allergan Style 410 implants (455 high cohesive and 195 soft touch gel implants). We used 35% Extra-Projected and 65% other implants. From September 2005 to December 2014, we implanted 185 Allergan Style 510 implants (77% in the sub-glandular position and 23% in dual-plane procedures). At a mean follow-up of 6.5 years, we observed Baker III-IV capsular contracture in 0.5% of cases, Baker II capsular contracture in 1.2% of cases, malpositioning or rotation in 0.7% of cases, and no infections, late seromas, or breast implant associated-anaplastic large cell lymphomas.
We are firmly convinced that our good outcomes derive from our commitment to minimizing implant contamination when positioning the prosthesis, following an accurate surgical technique. We always thoroughly consider the 14 clinical recommendations proposed by Deva et al. [19] when positioning a breast implant in order to minimize bacterial biofilm formation, avoiding periareolar incisions and dissection of the breast parenchyma, performing atraumatic dissection and minimizing devascularized tissues, performing pocket irrigation with antibiotics or betadine, minimizing implant handling, and performing intravenous antibiotic prophylaxis at the time of anesthetic induction [20-22].
Conclusions
The decision-making algorithm that we developed graphically summarizes the complex process behind a breast augmentation procedure and aims to help standardize decisions, basing them on quantifiable parameters and abandoning arbitrary and subjective assessment methods. Evidence-based surgery aiming to attain evidence-based outcomes mandatorily requires the scientific analysis of decision-making pathways.
We developed our decision-making algorithm using Allergan implants, but it could be easily adapted to any form-stable breast implant by a skilled user of other types of implants. Most implant manufacturers currently offer a wide choice of implant device dimensions, potentially enabling surgeons to obtain any result a woman may wish.
Long-lasting results are achieved by the best interactions between the implant and the patient’s tissues. In contrast, excessive volume, excessive projection, and the wrong implant pocket position occur as a result of improper interactions between the implants and the patient’s tissues. The surgeon’s aim should be to tailor the breast augmentation procedure for each individual woman. Moreover, the optimal breast augmentation procedures involve teamwork, in which the surgeon provides the best preoperative patient education with patient decision support devices and informed consent processes in which the consent is truly informed [6,23].
While the proposed algorithm standardizes decision-making pathways, at the same time, it provides an opportunity for the surgeon to consider the patient’s requests, which will definitively determine the final outcome.
We would like to underscore the inherent complexity of the breast augmentation decision-making process. Only by pursuing a standardized decision-making process and by performing accurate surgical procedures, aiming to reduce trade-offs and to minimize contamination (which does not necessarily mean longer operative times), with a tight-knit and “oiled” surgical team, could we aspire to obtain the best, most tailored, and longest-lasting results.
All aesthetic breast surgeons should analyze their own practice in order to standardize measurements and to understand exactly how measurements determine implant size and how the type of implant used impacts the measurement techniques in their experience. A national register with compulsory reporting of all breast augmentations with implant and outcome data would help obtain a complete picture of the various methods of preoperative planning and their impacts on patients’ outcomes [24,25].
Notes
No potential relevant to this article was reported.
Patient consent
The patient provided written informed consent for the publication and the use of their images.
