Safety of long-term subcutaneous free flap skin banking after skin-sparing mastectomy
Article information
Abstract
Background
A persistent problem in autologous breast reconstruction in skin-sparing mastectomies is skin restoration after skin necrosis or secondary oncological resection. As a solution to facilitate reconstruction, skin banking of free-flap skin has been proposed in cases where the overlying skin envelope must be resected, as this technique spares the patient an additional donor site. Herein, we present the largest series to date in which this method was used. We investigated its safety and the possibility of skin banking for prolonged periods of time.
Methods
All skin-sparing mastectomies and immediate autologous breast reconstructions from December 2009 until June 2013 at our institution were analysed.
Results
We identified 31 patients who underwent 33 free flap reconstructions in which skin banking was performed. Our median skin banking period was 7 days, with a maximum duration of 171 days. In 22.5% of cases, the banked skin was used to reconstruct overlying skin defects, and in 9.6% of cases to reconstruct the nipple-areolar complex. Microbiological and histological investigations of the banked skin revealed neither clinical infections nor malignancies.
Conclusions
In situ skin banking, even for prolonged periods of time, is a safe and cost-effective method to ensure that skin defects due to necrosis or secondary oncological resection can be easily reconstructed.
INTRODUCTION
Immediate autologous breast reconstruction after skin-sparing mastectomy is a well-established method with proven oncological safety [1]. However, a major unsolved challenge of this method is to reliably evaluate the perfusion of the mastectomy skin flap and the tumour-free status of the resected area intraoperatively.
Therefore, on the one hand, numerous clinical and technical methods have been described for evaluating the viability of the skin envelope, of which intraoperative indocyanine green (ICG) injection together with fluorescence imaging promises to be the most sensitive and practicable option [2].
On the other hand, with regard to oncological safety, intraoperatively performed frozen sections help to minimize the re-resection rate after skin-sparing mastectomies, especially when making a decision between the nipple- and skin-sparing methods [3]. Unfortunately, this technique is not ubiquitously available.
Nevertheless, although intraoperative evaluation methods predict skin necrosis more and more reliably, and re-resections are becoming less frequent, necrosis remains a relevant risk with potentially disastrous consequences. Even recent studies still report partial- or full-thickness skin flap necrosis rates in up to 78% of all cases [4].
In particular, postoperative skin or nipple necrosis due to excessive thinning of the skin envelope can often cause large defects with irreparable aesthetic consequences. Hence, multiple consecutive operations are often needed to restore the envelope using, for example, skin grafts with inferior haptic and aesthetic outcomes. Furthermore, they burden the patient with increased morbidity due to the need for an additional donor site, further operations, and greater costs, and ultimately may lead to a delay in receiving the needed chemotherapy or radiotherapy.
Subcutaneous in situ skin banking of free-flap skin paddles has been proposed as a feasible solution and as an effective method to ensure the possibility of quick skin defect repair in cases of borderline mastectomy skin flap perfusion or in patients with an elevated risk of secondary oncological resection. Nevertheless, to date, only case reports [5,6] and small series [7,8] have been published; these reports have demonstrated the advantages of short-term skin banking, but without a focus on safety or the prolonged use of this method.
To our knowledge, this is the first large series concerning skin banking in skin-sparing mastectomy, aiming to investigate the risk of infection and development of skin malignancy using this method. Furthermore, we evaluate the feasibility of skin banking for prolonged periods of time.
METHODS
A retrospective analysis was performed of all skin-sparing mastectomies and immediate autologous breast reconstructions at our institution from December 2009 to June 2013. All patients who underwent reconstruction with in situ skin banking were identified, their records were obtained, and relevant data were extracted.
All data presented in this article were obtained using our routine operative protocol, and all cases were treated according to our standard clinical approach. All patients provided informed consent for the surgical procedures.
Analysed parameters
The collected data included demographic information, as well as relevant clinical and laboratory findings such as minor and major complications, the banked skin surface area, the microbiological and histological work-up, and the duration of follow-up. Photo documentation, patient records, and operative reports were reviewed to identify any signs of infection.
Surgical procedure
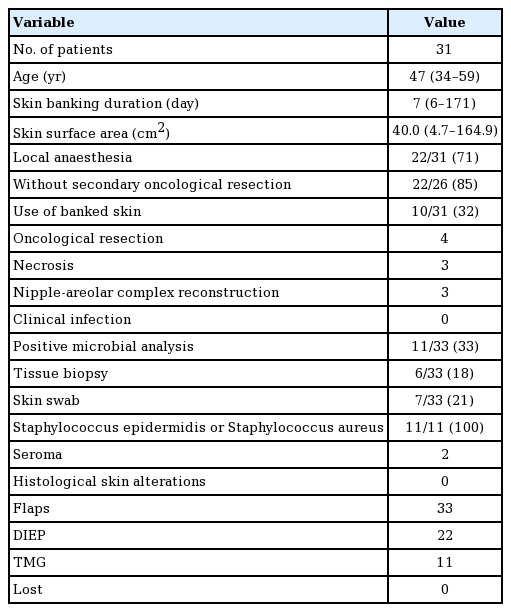
The skin banking procedure was different in nipple-sparing and skin-sparing mastectomies. In nipple-sparing mastectomies, the free flap was elevated and anastomosed to the internal mammary vessels. The standard incision used to perform mastectomy is a semicircular cut around the areola with a lateral extension. After successful anastomosis to the internal mammary artery and vein, the flap was inset and attached to the pectoral wall (Fig. 1A). The most peripheral areas of the skin of the free flap were then de-epithelialized, leaving the greater part of the central retro-areolar skin in situ, as well as the area behind the lateral incision (Fig. 1B).

Schematic illustration of the skin banking procedure
(A) The mastectomy was performed through a standard semicircular areolar incision with lateral extension. Afterwards, the flap was anastomosed to the recipient vessels (internal mammary artery and vein, IMA), inset, and sutured to the pectoral wall. (B) In nipple-sparing-mastectomies (NSM) a lateral monitor island (MI) was left visible for flap monitoring. The central banked skin was used if nipple-areolar complex reconstruction was required. (C) In skin-sparing-mastectomies (SSM) the flap was de-epithelialized in the peripheral areas, leaving the central skin banked. The banked skin could be used to reconstruct an areolar defect and in cases of lateral necrosis of the skin envelope, as well as to facilitate flap monitoring.
A small elliptical monitor island was defined and sutured in an “epidermis-on-epidermis” manner onto the lateral part of the lateral incision of the nipple-sparing mastectomy. The rest of the flap was completely buried, leaving the monitor island uncovered. The nipple-sparing mastectomy incision was then closed with standard skin sutures.
In skin-sparing mastectomies, a central circular monitoring skin island was defined and sutured in place, filling the areolar defect and leaving the rest of the skin of the central flap buried behind the skin-sparing mastectomy skin envelope (Fig. 1C).
All patients received a perioperative extended (72-hour) single shot of cefuroxime. During elective removal of the monitoring island or nipple reconstruction, the redundant subcutaneously banked skin was also de-epithelialized. During this procedure, an intraoperative skin biopsy and skin swabs were routinely performed, followed by histological analysis and a microbiological examination.
RESULTS
From December 2009 to June 2013, a total of 31 patients received a skin-sparing mastectomy and immediate autologous breast reconstruction with 33 free tissue flaps, respectively (Table 1).
Two patients received bilateral free flaps. Reconstructions were performed using deep inferior epigastric perforator (DIEP) flaps (n=22) or transverse musculocutaneous gracilis flaps (n=11). None of the flaps was lost. The median age of the patients was 47 years (range, 34–59 years).
Breast reconstructions were performed by multiple surgeons, and viability of the skin envelope was only assessed visually. The skin paddle of the flap was banked for a median period of 7 days (range, 6–171 days). The median skin paddle surface area was 40.0 cm2 (range, 4.7–164.9 cm2). In seven patients (22.5%) the banked skin was used to reconstruct a skin defect due to secondary oncological resection or skin necrosis of the mastectomy skin flap (Fig. 2). In three patients (9.6%), the skin was used to recreate the nipple-areolar complex (NAC).
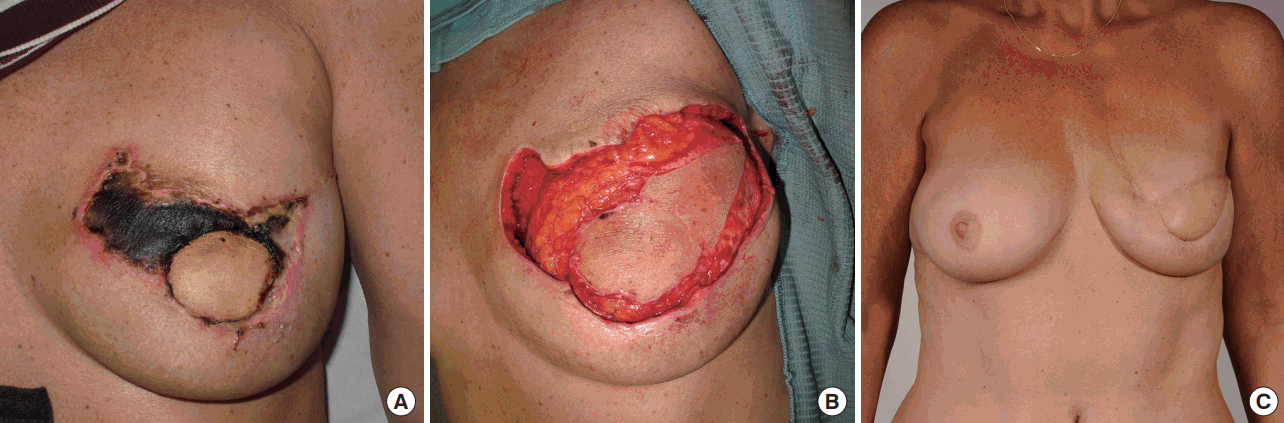
Clinical example
(A) Representative photo documentation of full-thickness skin necrosis 25 days after a skin-sparing mastectomy and immediate autologous breast reconstruction with a deep inferior epigastric perforator flap. (B) The subcutaneously banked skin was used to reconstruct the lateral skin defect. (C) Late postoperative result.
Retrospectively, no patient showed clinical signs of infection, although in 33% of patients, the microbiological analysis of either the cutaneous swabs or tissue biopsies came back positive for coagulase-negative Staphylococci (Staphylococcus epidermidis, Staphylococcus aureus). Intraoperatively, two patients showed mild seroma during removal of the banked skin paddle at 10 and 56 days postoperatively, respectively. In these patients, the microbiological examination was also positive for S. aureus.
The histological examinations showed no alterations of the banked skin or signs of malignancy. Only minor changes of the epidermis could be seen in one case after 56 days of skin banking (Fig. 3).

Histology of skin banking
Skin biopsy (hematoxylin and eosin staining), showing a thickened cornified layer with basket weave orthohyperkeratosis. Otherwise, the epidermis and junctional zone are inconspicuous (A, ×4). Prominent postcapillary venules are seen with perivascular lymphocytes, also involving the papillary dermis around the capillaries to a very moderate extent. Some minor erythrocyte clots are present within some vessels. No evidence is seen of abscess formation, necrosis, or malignancy (B, ×20).
In 85% of the patients, skin removal could be performed under local anaesthesia.
In five patients, secondary oncological interventions, such as lymph node dissections, were necessary under general anaesthesia. In these cases, the banked skin was removed during these interventions as well.
DISCUSSION
Intraoperative evaluation of the perfusion and viability of the skin flap after skin-sparing mastectomy is still a widespread challenge. Numerous methods for quantifying and solving this problem have been proposed, and although progress has been made, misinterpretation is still a problem [9]. Ultimately, it is still up to the surgeon to define viability and the resection margins, but “…the only true determinant of mastectomy skin flap survival is time” [10].
Despite advances in fluorescence imaging and ICG injections, this statement is still true, making it useful to implement some sort of delayed approach and to bank skin in order to have a backup when perfusion might be at risk. This is equally important for uncertain resection margins, especially in the retro-areolar area, where banked skin can be used to reconstruct a secondarily resected NAC without the burden of a second donor site.
In situ skin banking was first described by Kovach and Georgiad [7] using the transverse rectus abdominis flap, and has since been widely adopted [5,6]. The DIEP flap used in this series is considered to be the gold standard for autologous breast reconstruction and is the preferred flap worldwide [11,12]. Nevertheless, to our knowledge, the use of this flap for skin banking has not yet been described in any publications, although the DIEP flap is ideal due to the minimal donor site morbidity and the overabundance of skin compared to other autologous options. This makes very large buried skin flaps possible, without additional risk (as our data demonstrate).
Burying skin appears unnatural to many surgeons, not least because the skin is populated by a plethora of bacteria. However, in our study, tissue biopsies and skin swabs revealed only contamination with commensal skin bacteria, which caused no clinical signs of infection. Nevertheless, we found 2 seromas. Whether these were the results of a low-grade infection, as similar findings occur in low-grade infections after breast prosthesis implantation [13], remains unclear. Furthermore, we were not able to identify any studies in the literature discussing the microbiological colonization of buried skin to compare our findings with. In this context, it is worth mentioning that we used a prophylactic extended single shot of antibiotics (cefuroxime) for the first 72 hours.
When comparing tissue cultures with skin swabs, biopsies appeared to be more sensitive, confirming that tissue biopsy is the gold standard [14]. It is possible that bacteria hidden in skin appendages, such as hair follicles and glands, are protected from the immune system. This could explain why superficial swabs were not as sensitive as biopsies. Generally, microbiological investigations appear to be futile unless a clinical infection is diagnosed. In such cases, we recommend tissue biopsy.
Histologically, we found that even skin flaps that were buried for as long as 171 days revealed no relevant alterations or malignancies. In one patient, a biopsy obtained after 56 days showed a thickened cornified layer, but no further changes. Even inclusion cysts were not present, even though they are to be expected [15].
Sentinel skin paddles are routinely used for free-flap perfusion monitoring and are normally removed after several days under local anaesthesia. In most of our patients, the banked skin could also be removed without general anaesthesia, since neither the skin envelope nor the flap were sensate. However, this raises the question of how large one should design the buried skin island. We suggest that the surface area of the buried skin surface should at least equal that of the overlying NAC, since in most of our cases, we anticipated needing the buried skin flap to reconstruct the NAC, either due to necrosis or insufficient oncological safety. Generally, we suggest the larger the better, but the buried skin island should only be as large as can be comfortably removed under local anaesthesia.
No major additional stress or costs were generated by this 2-step procedure, especially since the monitoring skin islands had to be removed eventually. This procedure was particularly favourable in comparison to traditional skin reconstruction in cases of skin necrosis using split-thickness skin grafts, because they cause additional unsightly donor site morbidity.
In our series, we studied patients in whom skin flaps were buried for an exceptionally long period of time. This was either because the patients had personal obligations or had no interest in NAC reconstruction shortly after the initial operation. Previously, the maximum published period of time for a banked skin paddle was 6 days [6]. We showed that, if necessary for any logistical or patient-associated reason, skin flaps can stay buried for a prolonged period without any additional risk of histological alterations, epidermal loss, or infection.
Our findings confirm that in situ skin banking during immediate autologous breast reconstruction after skin-sparing mastectomy is a safe and cost-effective method that can be used, even for prolonged periods of time, to ensure that skin defects due to necrosis or secondary oncological resection can be easily reconstructed.
Acknowledgements
I thank Andrea Verstappen for her support in the writing of this manuscript.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.