Endoscopic transaxillary prepectoral conversion for submuscular breast implants
Article information
Abstract
Background
During breast augmentation, the transaxillary approach provides the advantage of allowing the mammary prosthesis to be placed through incisions that are remote from the breast itself, thereby reducing the visibility of postoperative scars. For patients experiencing capsular contracture who do not want additional scars, the previous transaxillary scar can be used for site change and implant exchange.
Methods
This study analyzed 17 patients (34 breasts) with submuscular breast implants with grade III-IV capsular contracture who received treatment from 2010 to 2015. The mean age of the patients was 29 years (range, 20–38 years). The inclusion criterion was a pinch test of more than 3 cm at the upper pole of the breast. Previous axillary scars were used to expose the pectoralis fascia, and submuscular breast implants were removed carefully. The dissection underneath the pectoralis fascia was performed with endoscopic assistance, using electrocautery under direct visualization.
Results
The mean follow-up period was 14 months (range, 6–24 months). The entire dissection plane was changed from the submuscular plane to the subfascial plane. Round textured gel implants were used, with a mean implant size of 220 mL (range, 160–300 mL). Two patients developed grade II capsular contracture. There were no cases of malposition or asymmetry. Three patients complained of minor implant palpability. None of the patients required additional surgery.
Conclusions
Endoscopic subfascial conversion may be an effective technique for treating capsular contracture and avoiding scarring of the breast in selected patients.
INTRODUCTION
Transaxillary submuscular breast augmentation is the most popular technique employed in East Asia for patients who wish to avoid additional scars in the aesthetic unit of the breast. Young Asian women with small breast volume, tight skin tone, and inconspicuous inframammary folds (IMFs) often opt to undergo breast enlargement. Additionally, scars made on the skin of Asians tend be less concealable than those made on the skin of Caucasians.
When capsular contracture occurs after breast augmentation, site change and implant exchange may play an important role in treatment. However, the data on capsulectomy and capsulotomy are still controversial [1-12].
In revision surgery of a previous transaxillary breast augmentation, additional scars often result, as it is necessary to proceed through the IMF or the periareolar route. However, if a patient does not want further scarring on her breasts, the surgeon can consider using the same transaxillary route to treat capsular contracture.
We sought to treat capsular contracture of previous transaxillary submuscular breast augmentation by site change and implant exchange through the same axillary scar in selected patients who wished to avoid an additional breast scar.
METHODS
A prospective study was conducted among 17 patients who underwent reoperative transaxillary subfascial conversion from February 2010 to February 2015. All the patients included in the study had submuscular breast implants with previous axillary scars. Those with a pinch test of less than 3 cm at the upper pole of the breast and less than 4 mm of thickness at the IMF level were excluded. All the patients had undergone previous breast augmentation more than 1 year before, and three of them had a surgical history of two or more reoperations, including capsulotomy and capsulectomy.
Patients with a past medical history of site change and repeated capsular contracture were excluded from the study. After ultrasonographic findings were evaluated, patients with abnormal features, such as late seroma or an intracapsular mass, were also excluded. The indications for the procedure were the patient’s unwillingness to have an additional scar on her breast and a pinch test of 3 cm or more at the upper pole. The surgeon counseled patients about the possible surgical approaches, and the patients provided informed consent. The dimensions of the implants were determined preoperatively by measuring the base width of the breast and the body morphology.
Operative technique
The entire surgical procedure was done using electrocautery dissection with direct endoscopic visualization. The endoscopic subfascial dissection was followed by the transaxillary removal of previous submuscular implants. After dissecting subfascially over the previous implant pocket, new implants were placed under the pectoral fascia.
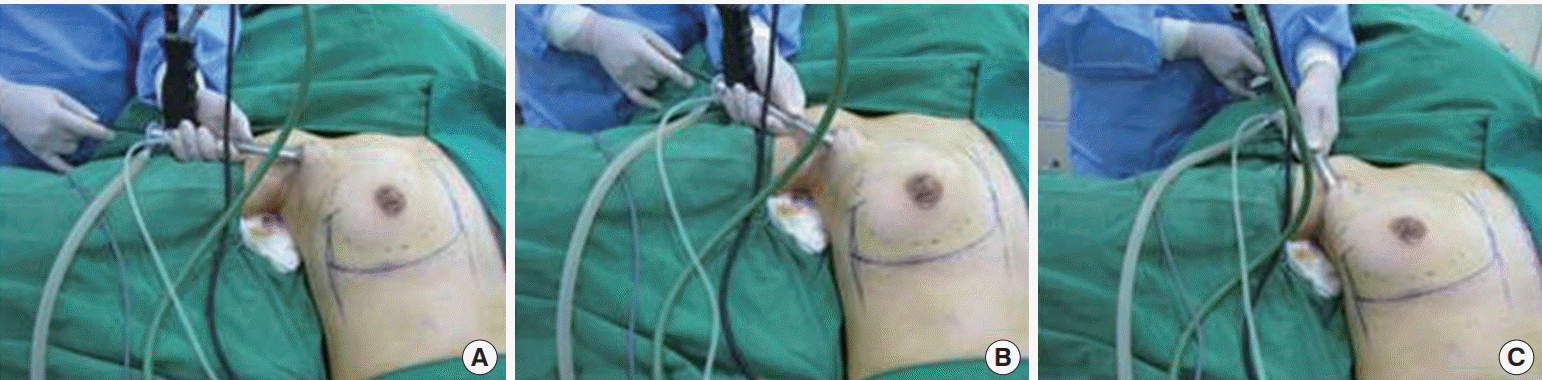
Under general anesthesia, patients were positioned supine with their arms abducted 90°. The incision through previous axillary scars and the 3-cm subcutaneous dissection reached the lateral border of the pectoralis major.
Step 1: removal of previous implants
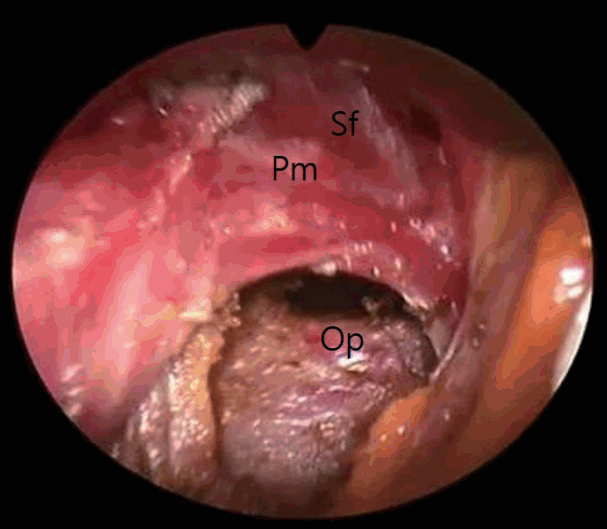
When the pectoral fascia was opened, the layer between the pectoralis major and minor could be accessed. Hemostasis of the lateral thoracic vessels was achieved. After opening the implant capsule, the breast implants in the submuscular space were carefully removed and bleeding spots were controlled under an endoscope (Richard Wolf GmbH, Knittlingen, Germany). It was often necessary to coagulate granulation tissue or to modify the capsule by electrocautery. Usually, closing the opening of the submuscular pocket was not required (Fig. 1).
Step 2: subfascial dissection, superior and medial areas (zone 1)
Subfascial dissection was then started using sharp cautery under direct visualization as far as the visual field allowed, and an endoscope was introduced with a straight needle-tipped electrocautery device. Under magnification, electrocautery dissection proceeded carefully, preserving the glistening pectoral fascia overlying the yellow subcutaneous fat. Blunt dissection was strictly avoided. Overall, the dissection procedures were performed in the cutting mode of cautery, and the occasional bleeding points were controlled by prospectively using the coagulation mode (Fig. 2).
At first, dissection started from the superomedial area and proceeded to the inferior and lateral areas in a clockwise fashion on the right breast sequentially (Figs. 3, 4). Care was taken when positioning the endoscope to avoid fascial penetration, especially along medial boundaries. When necessary, parts of muscle bundles were intentionally attached to the fascia. The fascial structures were elevated as much as possible from the underlying exposed muscle fibers to make an envelope thick enough to cover the implants. Excessive medial dissection was avoided to leave an intermammary distance of at least 3 cm.
Step 3: subfascial dissection of the inferior area (zone 2)
The endoscopic subfascial dissection was simple and straightforward, as there was less chance of encountering larger blood vessels in this space than there was in the submuscular space. Similarly, at the inferior border, the dissection was consistently continued downward to the predetermined IMF markings. However, in cases of low-lying implants or high costal origin, the dissection plane was carefully maintained above the implant capsule to avoid communication with the submuscular space (Fig. 5).
Step 4: subfascial dissection of the lateral area (zone 3)
Usually, the pectoral fascia enclosing the pectoralis major is not in the same plane as that of the deep fascia of the serratus and external oblique muscles. Therefore, dissection underneath the pectoral fascia could not be carried out continuously lateral to the pectoralis major, and a deeper dissection under the deep fascia layer was necessary to maintain a consistent envelope. Consequently, if the base diameter of the implant exceeded the limit of the lateral pectoral edge, the subfascial dissection could not be carried out as a perfect continuous layer, and interruption of the single plane beyond the lateral border of the pectoralis muscle was inevitable.
Under such circumstances, the superficial fascia of the serratus anterior was elevated to maintain the thickness of the lateral envelope under direct endoscopic control, taking care to avoid neurovascular injury to the intercostal bundles. Blunt-force dissection in this area frequently results in damage to the serratus muscle and to the vessels and nerves of the intercostal bundles, and the natural curvature of the inferolateral breast is likely to be destroyed (Fig. 6).
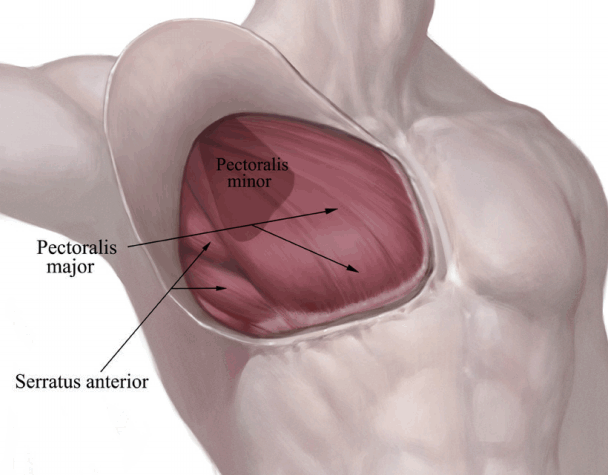
Subfascial dissection of the lateral area
Usually, the pectoral fascia enclosing the pectoralis major is not in the same plane of the deep fascia of the serratus and external oblique muscles. Therefore, the dissection underneath the pectoral fascia could not proceed continuously lateral to the pectoralis major.
After the pocket was made, bipolar forceps were used to stop bleeding points. The pocket was then irrigated with a solution of povidone-iodine, gentamicin, and normal saline [13]. After checking the size and shape of the pocket using disposable sizers, round textured gel implants were inserted through a Keller Funnel 2 delivery device (Keller Medical Inc., Stuart, FL, USA). The fascial layers were closed, followed by subcutaneous and skin closure with absorbable sutures, and a light dressing and an Ace bandage were applied over the armpit area. All patients were allowed to shower starting 2 days postoperatively and to resume light activity starting 3 days postoperatively. Generally, it was possible for the patients to perform weight-bearing exercises of the upper limbs starting 4 weeks postoperatively.
RESULTS
A total of 17 women underwent transaxillary subfascial conversion with endoscopy. The demographic data of the women were as follows: mean age, 29±9 years; mean height, 164.5 cm; mean weight, 51.7 kg; and mean body mass index, 19.1 kg/m2.
Thirty-four submuscular implants were removed, of which 20 were textured-surface, round breast implants, 10 were smooth-surface round implants, and the remaining 4 were shaped gels. Of the 24 textured implants, 12 were Allergan Biocell, 8 were Mentor Siltex, and 4 were Sientra products. All smooth-surface implants were Allergan products (Inamed). The mean volume of the removed implants was 254 mL (range, 175–350 mL).
Thirty-four round textured gel implants were placed (Sebbin, France). The mean volume of the new implants was 220 mL (range, 160–300 mL).
The mean follow-up period was 14 months (range, 6–24 months). No major complications such as severe bleeding, infection, breast implant rupture, or severe asymmetry were observed. No severe deformities due to implant rotation or displacement were detected. Animation deformity or severe chest pain was not found. Unilateral Baker grade II capsular contracture developed in two patients (11.8%) at 1 to 6 months postoperatively. Three patients complained of minor implant palpability. None of the patients required any additional surgery during the follow-up period (Figs. 7, 8).
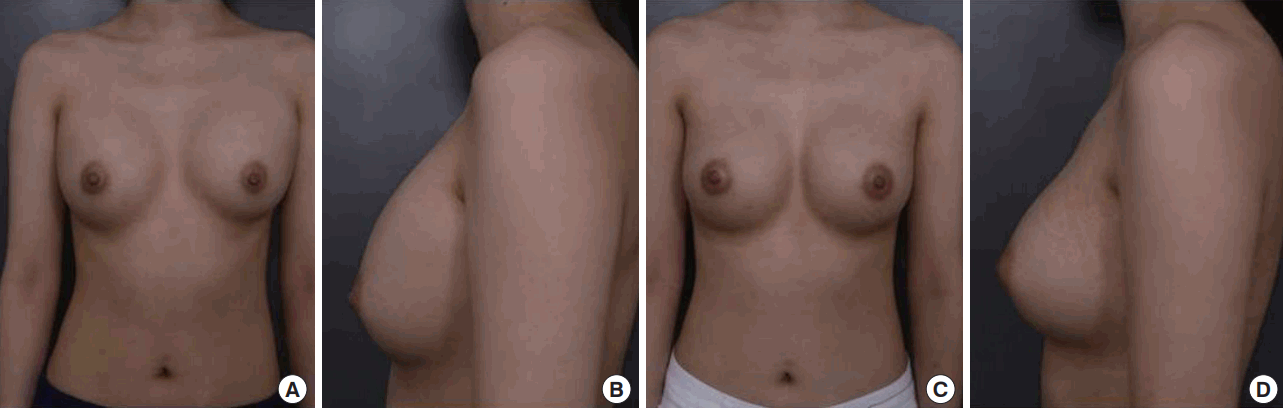
A 27-year-old female patient
A 27-year-old female patient (165 cm, 49 kg, body mass index=18 kg/m2 ). (A, B) Preoperative photographic findings of the patient. She had grade IV capsular contracture after previous transaxillary submuscular breast augmentation 2 years ago (Sientra, shaped gel moderate 275/275). (C, D) Postoperative photographic findings of the patient, 1 year after the subfascial site change and implant exchange through same transaxillary route (Sebbin, textured round gel 280/280).

A 34-year-old female patient
A 34-year-old female patient (168 cm, 53 kg, body mass index=18.8 kg/m2). (A, B) Preoperative photographic findings of the patient. She had grade III capsular contracture and implant malposition, including symmastia, after transaxillary submuscular augmentation 22 months previously (Mentor, round textured moderate plus 300/300). (C, D) Postoperative photographic findings of the patient, 1 year after transaxillary subfascial conversion (Sebbin, round textured gel 300/300).
DISCUSSION
The transaxillary approach is the most popular breast augmentation technique in East Asia. Usually, submuscular pockets are created for patients with sparse breast tissue, and round or shaped implants are placed using blunt dissection or the endoscopic technique. Different types of implants can be customized for individual patients to produce optimal results.
Site change and implant exchange have recently been proposed as important options for treating capsular contracture [1-12]. The treatment algorithm of capsulectomy, site change, and implant exchange is only partially supported by clinical evidence. Although site change and implant exchange are associated with reduced capsular contracture recurrence rates, the data on capsulectomy are less conclusive. For this reason, in this study we endeavored to manage capsular contracture by site change and implant exchange. Moreover, since breast capsular contracture is often accompanied by asymmetry or implant malposition, site change can be a good option to correct these issues as well (Fig. 8) [14].
However, most revision operations are performed through the inframammary or periareolar routes, which leave multiple unsightly scars on the chest. For patients who desire to avoid additional scars in the aesthetic unit of the breast, surgeons must utilize the same transaxillary approach to treat capsular contracture. In such circumstances, the only option might be to employ an endoscopic technique to visualize the internal tissues and to identify the correct plane for site change and implant exchange.
When breast capsular contracture occurs after subglandular augmentation, new pockets can be made under the pectoralis muscle, with or without capsulotomy or capsulectomy. This procedure can be accomplished using the transaxillary approach, which easily supplies enough muscular padding. For submuscular breast implants with enough soft tissue padding, it is possible to change the sites and implants through the same axillary canals. Additionally, the procedure can be executed in elaborate detail under direct endoscopic control. With the advent of precise endoscopic techniques, the creation of pockets with adequate dimensions and with symmetric IMFs through the transaxillary approach has become more reliable [15-17].
The superficial pectoralis fascia covers the outer part of the pectoralis major, running caudally until the IMF, and terminates on the rectus sheath. It defines the IMF as a true osseocutaneous ligament (Fig. 6). The deep pectoralis fascia is deep to the pectoralis major and terminates at its inferior border [17,18]. At the lateral border of the pectoralis major, the superficial and deep pectoralis fascia are united, becoming the axillary fascia, which continues laterally to the latissimus dorsi. If the superficial pectoralis fascia plane is anatomically followed below the inframammary fold, it will be terminated at the subcutis, because the superficial pectoralis fascia disappears into the rectus abdominis fascia at this level. For this reason, a subfascial pocket should be dissected sharply rather than bluntly [19]. Blind dissection can make the skin very thin or destroy the definition of the IMF. Maintaining consistent envelope thickness and the natural curvature of the IMF in the inferolateral region is only possible with sharp dissection under direct visualization.
Several articles have described mixed subglandular and subfascial dissection [20], which started from the lateral border and proceeded down to the sixth intercostal space, where the junction of the pectoral, rectus abdominis, and external oblique muscle fasciae was found. In this location, the plane shifted from the subfascial to the subglandular layer. Undermining of this plane was completed at 2 cm below the IMF.
However, despite making a thin envelope, it would be better to continue the subfascial dissection downward consistently, without changing the plane. When the base diameter of the implant was wider than the span of the pectoralis major fascia, the deeper fascial layer was utilized to add thickness to the implant envelope, especially in the inferior and lateral regions. Previous studies have documented that the total subfascial technique could reduce the risk of capsular contracture [21,22].
Transaxillary subfascial breast augmentation leads to cohesive and satisfactory results with easier dissection. In comparison with submuscular placement, this procedure has less risk of hematoma and pain, and enables faster recovery with less likelihood of intercostobrachial nerve injury. Animation deformity does not occur with muscle contracture. Although the full-thickness pectoralis muscle ensures significant long-term soft tissue coverage, subfascial coverage can supply enough padding over the implants in cases of supple pinch thickness. Although the fascia (0.2–1.0 mm) is not a thick tissue, it is relatively inelastic in comparison with the skin and muscle. This helps to avoid implant deformation during motion, leaves an additional soft tissue interface, and minimizes the implant edge prominence with less morbidity [22].
A planned dissection sequence is important while performing subfascial dissection in order to create a sufficiently-sized clear visual field and to control bleeding promptly. Sticking to the sequence minimizes unnecessary motion and promotes faster operations [23].
In this study, we placed textured round gel implants through the axillary approach, which results in more predictable aesthetic outcomes postoperatively compared to smooth implants because of tissue adherence. Moreover, it has been reported that capsular contracture develops approximately 5 times more frequently with smooth implants than with textured implants. When a reoperation is performed, most patients want to reduce the size of the implants to obtain a better feel and a more natural appearance [24].
In this study, we treated breast capsular contracture in selected patients with a pinch test of 3 cm or more at the upper pole, using endoscopic subfascial dissection to change the site and exchange the implants through the previous transaxillary route. We employed an endoscopic technique to create bloodless operating fields with minimal trauma and to facilitate the elaborate dissection of the new plane. However, for revision surgery, we acknowledge that it is easier and more reliable to use the IMF or periareolar approach if the patient agrees to the creation of additional scars.
In the first stages of a submuscular implant procedure, it is important to conduct a careful internal examination. If abnormal signs of an unhealthy capsule are found, such as extensive granulation tissue, an intracapsular mass, degenerative changes with a seroma, or calcification, a total capsulectomy should be performed, and it might be best to abandon the plane conversion.
Endoscopic surgery has some disadvantages, including the need for special equipment and a steep learning curve. The operating time tends to be longer than the blind technique, and reoperations can be difficult. However, for selected patients who do not want additional scars on their breasts, the same route could be utilized to perform site change and implant exchange to treat capsular contracture. For submuscular breast implants, site change to the subfascial space was successfully performed when the upper pole pinch test was over 3 cm. The thickness of the soft tissue is an important issue in the subfascial plane procedure. If the pinch test has a result of less than 4 mm at the IMF or less than 3 cm at the upper pole, the surgeon should avoid subfascial plane surgery. Generally, the transaxillary approach is not recommended for women with glandular ptosis or constriction of the lower pole, or for difficult reoperations with repeated capsular contracture or late seroma [23,25].
In conclusion, 17 women underwent reoperative transaxillary subfascial plane conversion under direct endoscopic visualization. The aim of this procedure was to treat capsular contracture by site change and implant exchange, while avoiding additional breast scars. Round textured gel implants were used in the subfascial plane. In our opinion, endoscopic transaxillary site change can be an excellent tool for young Asian women with previous axillary scars who wish to avoid an additional scar in the aesthetic unit of their breast.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.