Immediate breast reconstruction following nipple-sparing mastectomy in an Asian population: Aesthetic outcomes and mitigating nipple-areolar complex necrosis
Article information
Abstract
Background
Nipple-sparing mastectomies (NSMs) are increasingly performed to obtain the best aesthetic and psychological outcomes in breast cancer treatment. However, merely preserving the nipple-areolar complex (NAC) does not guarantee a good outcome. Darkly pigmented NACs and a tendency for poor scarring outcomes are particular challenges when treating Asian patients. Herein, we review the reconstructive outcomes following NSM at Singapore General Hospital.
Methods
All breasts reconstructed following NSM over an 11-year period from 2005 to 2015 were reviewed. Information was collected from the patients’ records on mastectomy indications, operative details, and complications. Patient satisfaction, breast sensation, and aesthetic outcomes were evaluated in 15 patients. Sensation was quantified using the Semmes-Weinstein monofilament test.
Results
A total of 142 NSMs were performed in 133 patients for breast cancer (n=122, 85.9%) or risk reduction (n=20, 14.1%). Of the procedures, 114 (80.2%) were autologous reconstructions, while 27 (19.0%) were reconstructions with implants. Complications occurred in 28 breasts (19.7%), with the most common complication being NAC necrosis, which occurred in 17 breasts (12.0%). Four breasts (2.8%) had total NAC necrosis. The overall mean patient satisfaction score was 3.0 (good). The sensation scores were significantly diminished in the skin envelope, areola, and nipple of breasts that had undergone NSM compared to non-operated breasts (P<0.05). Half of the subset of 15 patients in whom aesthetic outcomes were evaluated had reduced nipple projection.
Conclusions
Immediate reconstruction after NSM was performed with a low complication rate in this series, predominantly through autologous reconstruction. Patients should be informed of potential drawbacks, including NAC necrosis, reduced nipple projection, and diminished sensation.
INTRODUCTION
In the current era of breast cancer treatment, nipple-sparing mastectomy (NSM) is utilised with greater confidence as more evidence emerges to support its oncological safety [1,2]. With careful patient selection, NSM has been shown to confer comparable oncological outcomes to skin-sparing mastectomy, which is the current gold standard in breast cancer treatment [3]. With a recent increase in the public’s awareness of heritable breast cancer syndromes, patients who chose to undergo mastectomies are trending towards a younger age than the traditional age group in which breast cancer is diagnosed. In a recently published series from our institution, there was a greater than 10-fold increase in the NSM rate after 2006 (P<0.01) [4].
As a result of these trends, a paradigm shift has occurred in the management of breast cancer, from life-saving surgery to an approach that places an equal emphasis on aesthetic outcomes, quality of life, and oncological clearance. Preserving the breast skin envelope and nipple-areolar complex (NAC) is believed to result in the best aesthetic and psychological outcomes following a mastectomy. Despite its obvious advantages, NSM is prone to NAC necrosis, which potentially negates the benefit of preserving the NAC. In addition, patients may be disappointed with the outcomes if they are insufficiently prepared for possible changes in the position and appearance of the nipple and alterations in breast sensation.
There is a paucity of studies addressing the outcomes of reconstruction after NSM in Asian populations. Fundamental differences exist between Asian and Caucasian patients, and these differences must be considered when planning breast reconstruction in the former group. Such differences include the tendency towards poorer scarring outcomes, smaller breast size, highly pigmented areolas that are more conspicuous against the lighter-coloured breast skin, and a greater reluctance for prostheses and repeated procedures under general anaesthesia.
Peri-areolar, radial, inframammary, mastopexy, and transareolar incisions have been described for NSMs. The peri-areolar incision, which is often extended laterally or inferiorly for greater access to the breast, is commonly the incision of choice in Asian patients, as the eventual scar is well concealed in the dark-colored NAC. If an implant reconstruction is performed, the incision is closed primarily. In cases where autologous reconstruction is performed, a skin paddle is incorporated into the wound closure to allow the flap’s viability to be monitored and to accommodate tissue swelling that may compromise the mastectomy skin envelope.
In this study, we aimed to examine the outcomes of immediate breast reconstruction following NSM in an Asian population with autologous reconstruction as the predominant method of reconstruction, paying particularly close attention to NAC-related outcomes. To the best of our knowledge, ours is the largest Asian series analysing reconstructive outcomes following NSM to be published.
METHODS
The medical records of all patients who had undergone breast reconstruction following NSM performed by a surgeon at Singapore General Hospital over an 11-year period from January 2005 to December 2015 were reviewed. The study was approved by the SingHealth Institutional Review Board (IRB No. 201703-00148). Women who underwent NSM for cancer treatment or risk reduction were included in the study. Reconstruction was performed using autologous tissue, implants, or a combination thereof.
Basic patient demographics and the indication for NSM were obtained from the patients’ medical records, along with operative details, including the type of mastectomy incision and details of the reconstructive procedure. Postoperative complications were reviewed, with special attention to those involving the NAC.
At follow-up, aesthetic outcomes of the NAC were evaluated in a sample of 15 patients after patients’ consent was obtained. Nipple height was measured on lateral pre- and postoperative photographs to ascertain the loss of nipple projection. The NAC of the reconstructed breast was also compared to that of the contralateral breast to evaluate its position and the presence of any deformity. NAC and skin envelope sensation was tested using the Semmes-Weinstein monofilament test for fine touch, with filament sizes of 2.83, 3.61, 4.31, 4.56, and 6.65. The breast skin envelope, areola, and nipple were divided into quadrants and each of the 5 monofilaments was tested over the epicentre of each quadrant. If the patient was unable to detect the 6.65 filament, sensation was recorded as absent. Filament sizes were converted to categorical variables as follows: 0=no sensation, 1=6.65, 2=4.56, 3=4.31, 4=3.61, and 5=2.83. The sensation score in each quadrant was averaged to derive an overall sensation score for the breast envelope, areola, and nipple of each patient. In patients who underwent unilateral mastectomies, the contralateral breast was used as the control group for comparison. To assess patients’ satisfaction with their reconstruction, a questionnaire was used that asked patients to rate several factors relating to their NAC (namely, appearance, symmetry, colour, position, sensation, arousal, and texture). Patients were asked to rate each aspect as excellent, good, fair, or poor. These ratings were converted into categorical variables from 1 (poor) to 4 (excellent) for analysis.
All NSMs were performed by a qualified breast surgeon. When previous biopsies or breast-conserving surgery had been performed, most surgeons’ preference was to incorporate the old scar into the new incision. A separate incision for the treatment of the axilla was made at the discretion of the breast surgeon. An intra-operative frozen section of retro-areolar tissue was examined by a pathologist in all cases, and the NAC was sacrificed if tumour involvement was confirmed.
All reconstructions were performed by a separate team led by a qualified plastic surgeon. Autologous reconstruction was performed using free or pedicled abdominal flaps, pedicled latissimus dorsi (LD) flaps with or without implant augmentation, and free thigh flaps. The choice of reconstruction was determined pre-operatively by the plastic surgeon according to the patient’s body habitus, lifestyle, and preferences.
Data were analysed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA). The chi-square test or the Fisher exact test was performed to determine the correlation between categorical risk factors and NAC necrosis. The Student t-test was used to compare mean sensation scores between breasts that had undergone NSM and controls. P-values <0.05 were considered to indicate statistical significance.
Surgical technique
Upon completion of the NSM, the mastectomy skin flaps were assessed for thickness and threatened NAC viability, as evidenced by bruising, discolouration, and poor bleeding from the skin edges. When the mastectomy skin flaps were not excessively thinned-out and a healthy NAC was present, immediate primary closure was planned. After the flap was shaped and inset, the skin flaps were draped over the reconstructed breast and assessed for tightness of closure. When excessive tension was anticipated, a skin paddle from the flap was incorporated into the closure (Fig. 1). This skin paddle could be excised after the resolution of postoperative oedema. If no tension was encountered, primary closure without a skin paddle was performed, with the plan to release the sutures around the NAC should signs of necrosis develop.
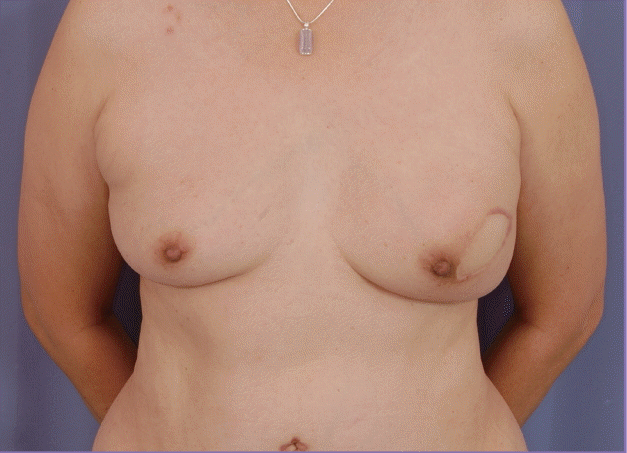
Immediate primary closure with skin paddle incorporation
This patient underwent immediate reconstruction with a pedicled transverse rectus abdominis myocutaneous (TRAM) flap. As excessive tension was encountered on primary closure, a skin paddle from the TRAM flap was incorporated to allow tension-free primary closure. Nipple projection, position, and nipple-areolar complex appearance were maintained at a 9-year follow-up.
If NAC viability was threatened, delayed primary closure was performed instead. The skin paddle of the flap was de-epithelized, save for an area slightly larger than the NAC or the area of the threatened skin envelope. The edges of the mastectomy wound were then tagged loosely over the “banked” skin paddle. The NAC was allowed to demarcate over the ensuing days. If the ischaemic insult was reversed and the NAC and skin flaps remained viable, the skin paddle was excised and primary closure was performed (Fig. 2). Again, a skin paddle was incorporated into the final wound if primary closure would have been performed under excessive tension (Fig. 3).
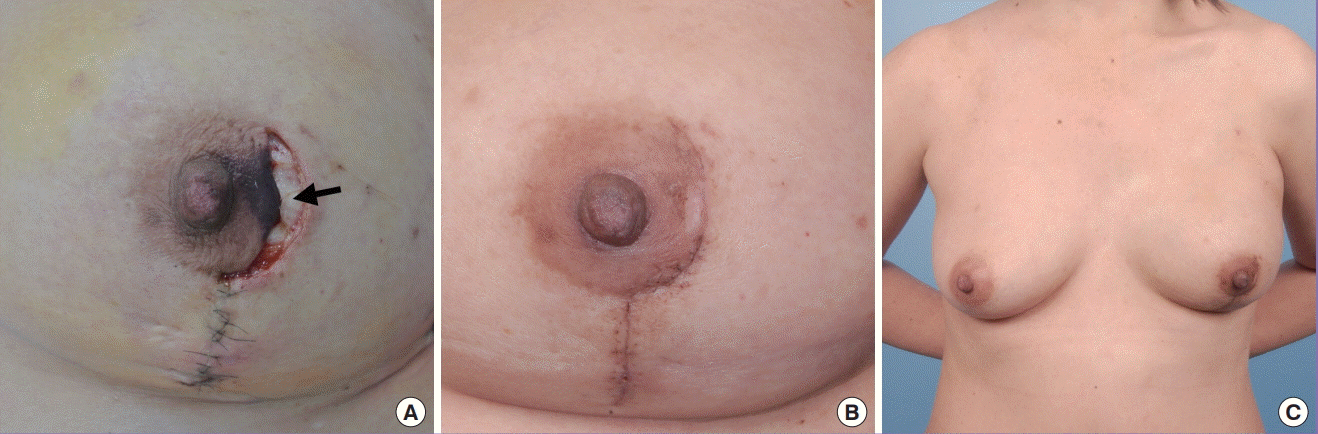
Delayed primary closure
In this patient, delayed primary closure was performed after the nipple-areolar complex showed signs of being threatened intra-operatively. (A) A disc of skin (black arrow) was banked under the mastectomy skin flaps and the nipple was kept with a slight stretch with loose tagging sutures to promote circulation in the subdermal plexus. (B) Two weeks later, the area of necrosis was superficial and complete skin closure was achieved after excising the banked skin. (C) Frontal view of the patient following reconstruction 6 months after surgery.
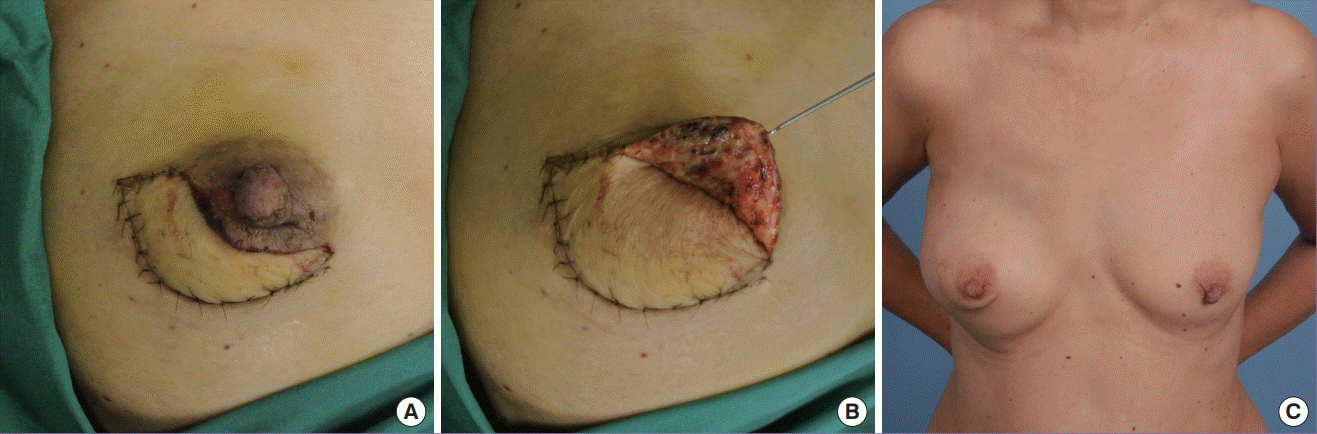
Delayed primary closure with skin paddle incorporation
(A, B) Delayed primary closure was performed in this patient who underwent immediate reconstruction with a free muscle-sparing transverse rectus abdominis myocutaneous flap, as nipple viability was doubtful intraoperatively. (C) Result 3 years postoperatively. The skin paddle was incorporated when the ischaemic insult had reversed. The skin envelope was too tight for direct closure.
Complete demarcation of NAC necrosis occurred after approximately 2 weeks. When partial necrosis of the NAC occurred, the necrotic tissue was excised and the banked skin flap was incorporated to provide adequate skin cover without causing asymmetry in the nipple position. If the NAC was completely sacrificed, the banked skin paddle provided robust, full-thickness skin that allowed nipple reconstruction to be performed in a symmetrical position to the contralateral NAC. The availability of a banked skin paddle also obviated the need for further skin graft donor sites.
If reconstruction with an implant was planned, a tissue expander was inserted into the subpectoral plane for a 2-stage reconstruction. Adequate muscle cover beneath any areas where the skin envelope was threatened was considered imperative. The best outcomes were achieved when subareolar tissue was preserved and no NAC necrosis occurred (Fig. 4).
Immediate primary closure with tissue expander insertion
This patient underwent a nipple-sparing mastectomy via a peri-areolar incision and 2-stage reconstruction with a tissue expander followed by a definitive implant 4 months later. (A) The subareolar tissue was well preserved (black arrow) and gradual tissue expansion was performed to allow perfusion of the skin flaps before definitive implants were inserted. (B, C) Good nipple position and projection were maintained at an 8-year follow-up.
Hyperbaric oxygen therapy (HBOT) was offered to patients in whom the viability of the entire NAC was threatened. Four patients were treated with HBOT, two (50%) of whom did not progress to full-thickness NAC necrosis that required surgical debridement.
RESULTS
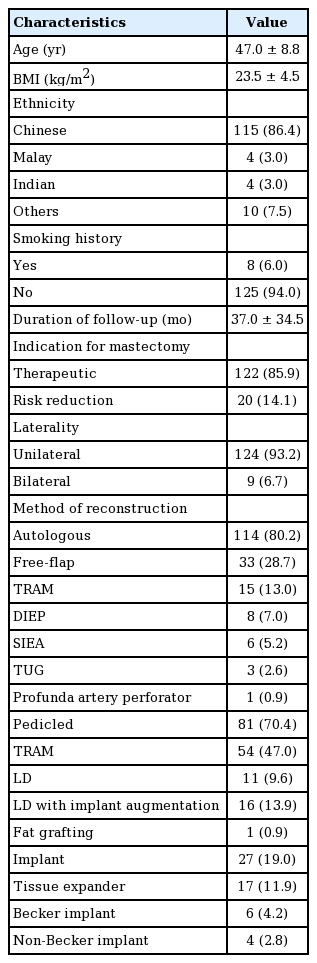
A total of 142 breasts in 133 patients were reconstructed over an 11-year study period (Table 1). The majority (n=115, 86.4%) were Chinese, while four patients (3.0%) were Malay, four (3.0%) were Indian, and 10 (7.5%) were of other ethnicities. Their mean age was 47.0±8.8 years. Of the patients, 124 (93.2%) underwent a unilateral NSM and nine (6.7%) had bilateral NSMs. NSM was performed for cancer treatment in 122 breasts (85.9%), and as risk reduction surgery in 20 (14.1%). The mean body mass index (BMI) was 23.5±4.5 kg/m2, and eight patients (6.0%) had a history of smoking. The mean duration of follow-up was 37.0±34.5 months.
Autologous reconstruction
Single-staged, autologous reconstruction was performed in 114 breasts (80.2%). The mean age of those who underwent autologous reconstruction was 47.8±7.9 years. These patients had a mean BMI of 24.0±4.3 kg/m2. In this group, 104 mastectomies (88.1%) were performed for breast cancer treatment, while the remaining 11 (9.6%) were for risk reduction. The mean duration of follow-up in this group was 34.6±33.0 months. Information on the type of incision was available for 102 mastectomies, of which 75 (73.5%) were performed via a peri-areolar incision and the remainder were performed using a radial incision. Thirty-three autologous reconstructions (28.7%) were performed with a free flap, of which 15 (13.0%) were muscle-sparing transverse rectus abdominis myocutaneous (TRAM) flaps, eight (7.0%) were deep inferior epigastric perforator flaps, six (5.2%) were superficial inferior epigastric artery flaps, three (2.6%) were transverse upper gracilis flaps, and one (0.9%) was a profunda artery perforator flap. Of the 81 pedicled flaps (70.4%), 54 (47.0%) were TRAM flaps, 11 (9.6%) were LD flaps, and 16 (13.9%) LD flaps were augmented with an implant. One breast (0.9%) was reconstructed with fat grafting alone.
Implant reconstruction
Twenty-seven breasts (19.0%) were reconstructed using an implant. Patients in this group had a mean age of 42.9±10.2 years and a mean BMI of 21.1±4.9 kg/m2. Eighteen breasts (66.7%) were reconstructed after cancer extirpation and the remaining nine procedures (33.3%) were risk-reducing mastectomies. This group had a mean follow-up duration of 47.4±39.0 months. Information on the type of incision was available for 23 mastectomies, of which 17 (73.9%) were performed through a peri-areolar incision, five (21.7%) through a radial incision, and one (4.3%) through an inframammary fold incision. Seventeen (63.0%) of the breast reconstructions were performed in 2 stages, with an initial tissue expander and subsequent definitive implant exchange. In six of these procedures (22.2%), immediate reconstruction using a Becker implant was performed, and the remaining four procedures (14.8%) involved single-staged reconstruction using a regular implant.
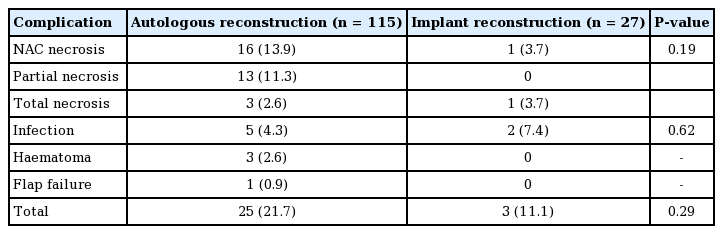
Surgical complications
Complications occurred in 28 reconstructed breasts (19.7%) (Table 2). Overall, necrosis of the NAC was the most common complication, occurring in 17 breasts (12.0%), followed by infectious complications in seven breasts (4.9%), including cellulitis of the skin envelope and superficial wound infections.
Of the breasts that underwent autologous reconstruction, 16 (13.9%) developed NAC necrosis. Partial NAC necrosis occurred in 13 breasts (11.3%), while complete NAC necrosis occurred in three (2.6%). Eleven patients (9.6%) required further surgical debridement, while the remaining four (3.5%) healed with regular dressing changes. One (0.9%) patient had her NAC excised after seeking a second opinion at a different institution. Of the breasts that required excision of a necrotic NAC and skin envelope, one case (0.8%) did not have an adequate amount of banked skin, and therefore required additional skin grafting (Fig. 5). There was a significantly higher rate of necrosis in those who had a free flap (n=8, 24.2%) than in those who did not (n=8, 9.8% P=0.04). Five breasts (4.2%) developed infectious complications, of which three (2.6%) required surgical drainage, while the rest resolved with antibiotics. Three breast haematomas (2.6%) occurred and required surgical drainage. One flap failure occurred (0.9%).
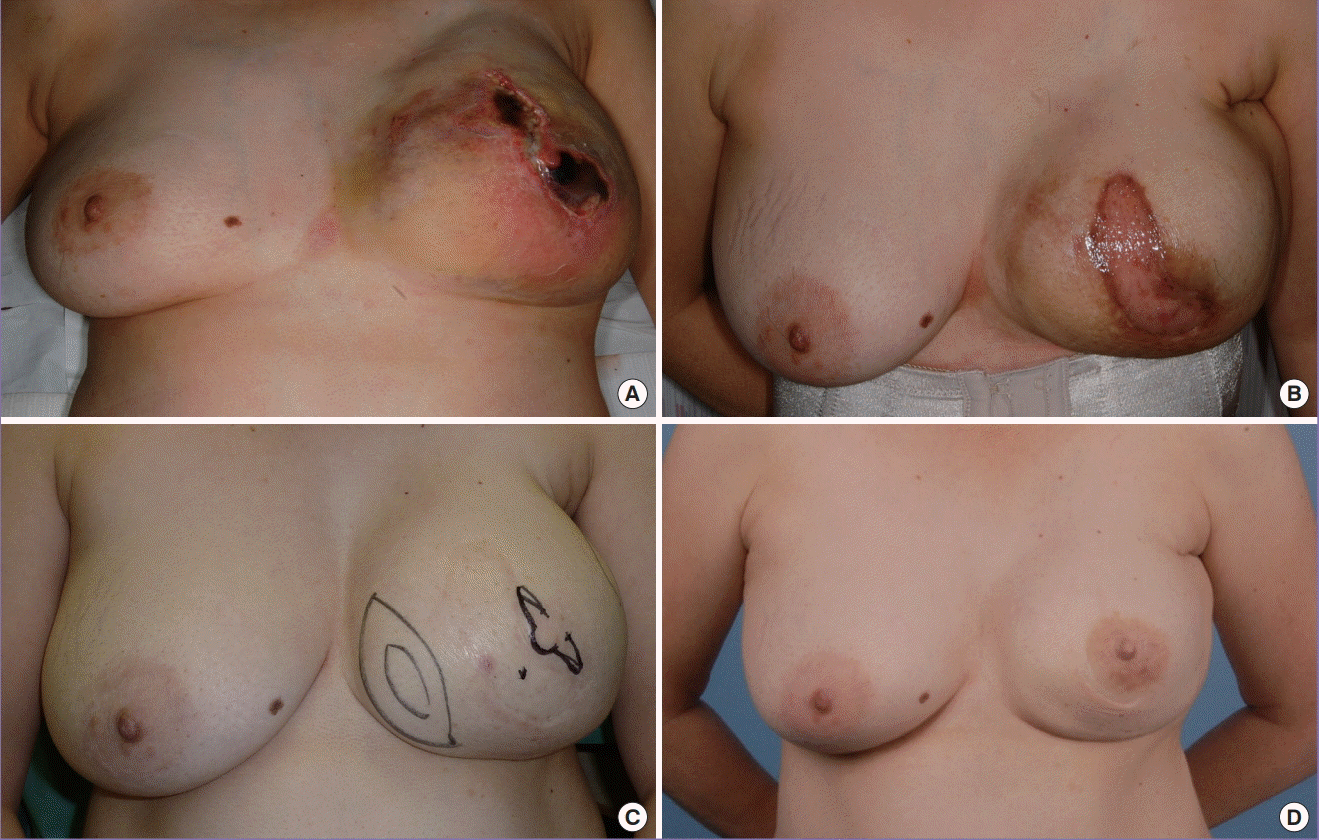
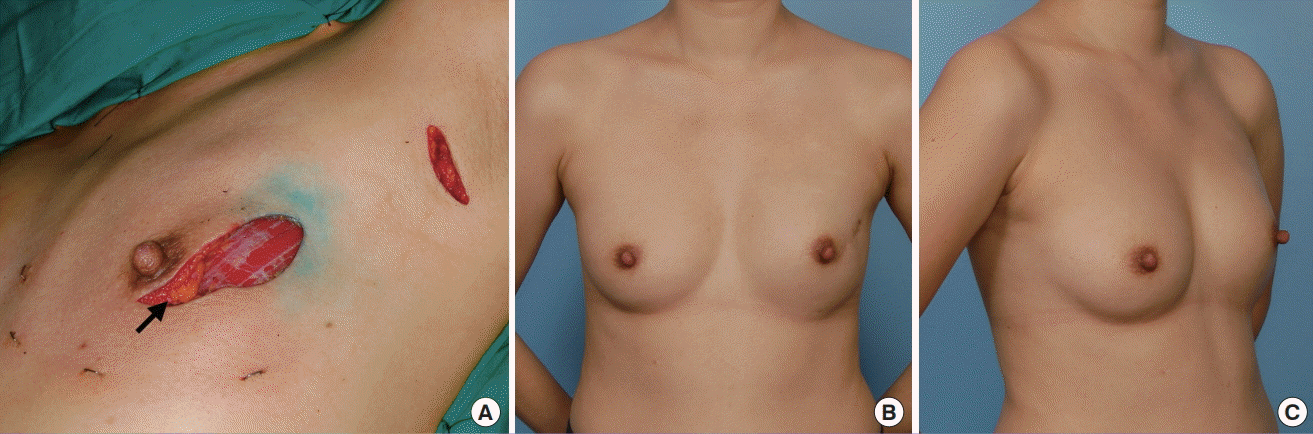
Nipple reconstruction after complete NAC necrosis
(A) Complete nipple-areolar complex (NAC) necrosis 2 weeks after a nipple-sparing mastectomy. (B) Owing to dermal preservation over the transverse rectus abdominis myocutaneous flap, split-thickness skin grafting was readily performed 2 weeks later. (C) Nipple reconstruction was accomplished with a modified C-V flap and a rolled dermal graft for support. It was placed off-centre to avoid the skin graft. Liposuction was performed over the lower inner quadrant of the breast to medialise the nipple. (D) Final outcome after nipple reconstruction 11 years postoperatively.
Infectious complications and NAC necrosis were the only complications in breasts that underwent implant reconstruction. Infectious complications occurred in two breasts (7.4%), while complete NAC necrosis occurred in one (3.7%), requiring eventual sacrifice of the NAC. One infected implant (3.7%) had to be removed.
Information on the incision type was available for 125 patients. NAC necrosis developed in 12 patients (12.9%) who had a peri-areolar incision and three patients (9.7%) who had a radial incision. In our study, peri-areolar incisions were associated with a higher rate of NAC necrosis than radial incisions, but this did not reach statistical significance (P=0.76). None of the patients with NAC necrosis were smokers. BMI and age were also not significant risk factors for NAC necrosis.
Appearance of the preserved NAC, patient satisfaction, and nipple sensation
NAC appearance, patient satisfaction, and nipple sensation were evaluated in a sample of 15 patients. The median duration of follow-up in this group was 103.4 months (range, 43.3–127.3 months). Although good symmetry in breast volumes was easily achieved in most patients, critical examination of the NAC revealed a degree of deformity in nine breasts (60%) when compared to the contralateral breast, contributing to a suboptimal aesthetic outcome. This included a loss of nipple projection (n=8, 53.3%), change in the colour of the NAC with a tendency for hyper-pigmentation (n=2, 13.3%), and nipple malposition (n=5, 33.3%) compared to the contralateral breast (Fig. 6).
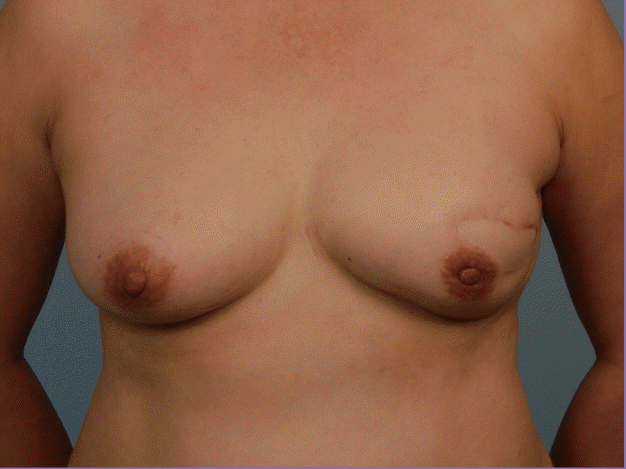
A contracted NAC with a retracted nipple
This patient underwent reconstruction with a single-staged ipsilateral pedicled transverse rectus abdominis myocutaneous flap. A small skin paddle was incorporated to reduce tension on the nipple-areolar complex (NAC) during closure, which was achieved primarily. Closer examination of the preserved NAC at a 7-year follow-up revealed a slightly contracted NAC with a loss of nipple projection.
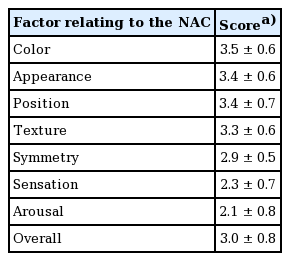
Patients rated the aspects of NAC appearance, colour, position, and texture with mean scores greater than 3 (good, with a score of 4 being “excellent”). However, symmetry, sensation, and arousal were rated as fair to poor by most patients, with mean scores of 2.9±0.5, 2.3±0.7, and 2.1±0.8, respectively (Table 3). The overall mean satisfaction score was 3.0±0.8.
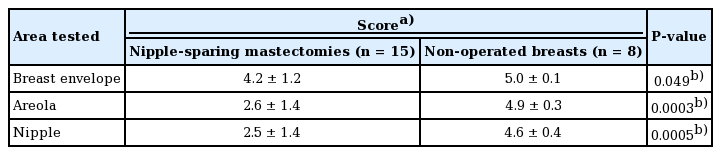
Sensation in the breast envelope, areola, and nipple of breasts that had undergone NSM was significantly reduced compared to the control group of non-operated breasts (Table 4). The mean sensation scores in breasts that had undergone NSM were 4.2±1.2, 2.6±1.4, and 2.5±1.4 in the breast envelope, areola, and nipple, respectively. In the control group, the corresponding values were 5.0±0.1 (P=0.049), 4.9±0.3 (P=0.0003), and 4.6±0.4 (P=0.0005).
DISCUSSION
NSM has gained popularity in recent years, as its oncological safety has been increasingly established [3]. Local recurrence occurred in five breasts (3.52%) in our study of 133 patients (142 mastectomies) with a mean follow-up duration of 37± 34.5 months. This rate is lower than has been reported in the literature, as local recurrence rates have been reported to have a weighted average of 11.4% in a recent meta-analysis [3]. A systematic review of NSMs performed in Asian populations has also shown that outcomes in Asian and Western populations are comparable in terms of local recurrence and nipple necrosis rates [5].
Although the purported benefits of preserving the NAC are obvious, there is a paucity of published studies addressing the concern that preservation of the NAC alone does not translate to satisfactory aesthetic outcomes. We believe that achieving satisfactory aesthetic outcomes is even more challenging in Asian patients than in Caucasian patients for the following reasons: (1) Asians have less aesthetically satisfactory wound healing than Caucasian patients, with a tendency for hyper-pigmentation and scar formation [6,7]; (2) Asian women have darker NACs, causing deformities to be more conspicuous; and (3) Asian women are more reluctant to undergo multiple procedures. With these considerations in mind, we critically examined the outcomes of breast reconstruction following NSM at our institution.
Asian patients are generally more conservative, with a preference for autologous reconstruction with a single-stage procedure (constituting 80.7% of patients in our study) rather than reconstruction with implants. This tendency is in contrast to large studies in Caucasian populations, where most reconstructions were performed using prostheses [8,9]. In the study of Casella et al. [8], 92.2% of patients underwent reconstruction with a prosthesis, of whom 64.6% required 2-stage surgery with a tissue expander followed by a definitive implant. This was the case for only 11.7% of the patients in our study. NAC necrosis is more readily minimised in cases of implant reconstruction, especially in 2-stage reconstructions where tissue expansion is accomplished gradually. Becker implants have not gained traction in our practice because of the need for precise pocketing.
Autologous reconstruction has been associated with a higher rate of NAC necrosis than implant reconstruction [10]. Although this was also the case in our study, this difference did not reach statistical significance. A pooled analysis by Endara et al. [10] showed the nipple necrosis rate to be 17.3% in patients who underwent autologous reconstruction. The rate of nipple necrosis in our series was 13.9%, and three patients (2.6%) had total NAC necrosis. The higher rate of nipple necrosis in autologous reconstructions may be explained by excessive tension on the skin envelope as a result of oedema and immediate filling of the breast envelope with the final volume of an autologous flap. This is compounded by inadvertently thinned-out skin flaps and suboptimal tissue handling, which may disrupt the blood supply to the skin pocket and NAC. With the use of delayed skin closure, we were able to mitigate these factors.
Amongst those who underwent autologous reconstructions, we observed a significantly higher rate of NAC necrosis in patients who had free flaps compared to those who had pedicled flaps (P=0.04). We hypothesise that this is due to the fact that the internal mammary artery was sacrificed as a recipient site for microvascular anastomosis, further de-vascularising the breast pocket. This is a plausible explanation in our series, as 75% (n=6) of the patients with NAC necrosis had flaps that were revascularized using the internal mammary artery. Another contributing factor may have been the prolonged folding and traction on the skin envelope required for surgical exposure of the internal mammary vessels.
In our study, the incidence of NAC necrosis was higher in patients who had peri-areolar incisions than those who had radial incisions, although the difference did not reach statistical significance. This trend is in keeping with the tendency observed in both animal studies and large retrospective series for peri-areolar incisions to be associated with reduced perfusion to the NAC and higher NAC necrosis rates [11,12]. In a retrospective review of 500 patients, Colwell et al. [12] found full-thickness incisions around the NAC to be a significant risk factor for NAC necrosis, mastectomy skin flap necrosis, and total complications. The peri-areolar incision is still used widely in our practice, as it is well concealed and allows easy access to all 4 quadrants of the breast. To reduce the risk of developing NAC necrosis, we now limit the extent of the peri-areolar incisions to less than half the NAC circumference, extending the incision inferiorly or laterally as required.
The rate of nipple necrosis in our population was admittedly high, although this figure included all cases of NAC necrosis, regardless of whether it was partial- or full-thickness, or whether the entire NAC was affected. Of the cases in our study, 9.6% required surgical debridement of a necrotic NAC and 2.8% had complete NAC necrosis. As we overcame the learning curve, we found delayed primary closure to be effective for salvaging threatened NACs. Various methods have been described to mitigate NAC necrosis. These include banking the NAC as a free graft [13], adding a delay procedure prior to NSM [14], and banking a skin paddle from the autologous flap beneath the mastectomy flaps [15,16]. We prefer the skin banking method, as it permits prompt healing by primary intention, thus avoiding a nonhealing wound during chemotherapy. Future nipple reconstruction is also possible with a preserved skin paddle.
Breast ptosis poses a greater challenge to achieving a symmetrical reconstruction, although few cases of ptosis were observed in our study population. These patients were either offered an autologous reconstruction, which allowed the creation of ptosis identical to the contralateral breast, or a simultaneous contralateral mastopexy. None of the patients in our series desired an intervention on the contralateral breast, and all patients with ptosis underwent autologous flap reconstruction.
All previous breast biopsy or wide excision scars were excised at the time of the NSM. The incision was planned in the same way as for a skin-sparing mastectomy [17]. Radial biopsy incisions were incorporated as a lateral extension of the peri-areolar incision. If the previous circumareolar incision was more distant than 1 areolar diameter from the NAC, this scar was excised through a separate incision. When autologous reconstruction was performed, the previous incision often facilitated the inclusion of a skin paddle into the wound closure. This prevented excessive tension on the skin envelope and distortion of the NAC [17]. None of the patients in our series received radiation therapy prior to NSM.
NAC ischaemia can result in depigmentation, loss of projection, and a contracted NAC from scar formation. These outcomes reduce the benefit of NAC preservation. It is thus crucial for the reconstructing surgeon to evaluate the skin flaps after the resection is complete and to adjust the reconstruction accordingly to minimise NAC necrosis. In our sample, 60% of patients had a NAC deformity, most commonly a loss of projection that resulted from coring of the subareolar tissue. These deformities were corrected using either dermal struts fashioned from a dermal C-V flap raised from the underlying autologous flap or internal cerclage sutures that have been described for nipple inversion correction [18].
The overall mean patient satisfaction score was 3, which corresponded to a “good” rating. Of note, patients were dissatisfied with aspects pertaining to symmetry, sensation and arousal, consistent with other large studies with long-term follow-up [19]. We found that sensation was significantly reduced in breasts that had undergone NSM, especially in the areola and nipple. We recommend that patients be counselled preoperatively about the possibility of reduced sensation.
Fluorescein dye angiography and laser-assisted indocyanine green (ICG) angiography have been used to evaluate the viability of the mastectomy skin envelope and NAC after mastectomy. Laser-assisted ICG angiography has been shown to be 100% specific and superior to both clinical judgement and fluorescein dye angiography in predicting mastectomy skin flap necrosis. These modalities, however, over-predicted the area of necrosis in 13.3% of necrotic breasts when laser-assisted ICG angiography was used and in 30.18% of necrotic breasts when fluorescein dye angiography was used [20]. Our preference is to give the skin flaps and NAC a chance for reperfusion by delaying closure, rather than excising an excessive amount of mastectomy skin and native NAC.
The primary limitation of our study is its retrospective nature, which precluded the analysis of other risk factors for NAC necrosis such as breast volume, ptosis, and the thickness of the mastectomy skin flaps. Additionally, breast sensation and patient satisfaction evaluations were limited to a subset of our patients. We intend to prospectively examine our outcomes with a standardised protocol in future studies.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the SingHealth Institutional Review Board (IRB No. 201703-00148) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.