3-Dimensional fasciectomy: A highly efficacious common ground approach to Dupuytren’s surgery
Article information
Abstract
Background
Numerous Dupuytren’s fasciectomy techniques have been described, each associated with unique surgical challenges, complications and recurrence rates. We describe a common ground surgical approach to Dupuytren’s disease; 3-dimensional fasciectomy (3DF). 3DF aims to address the potential contributors to the high recurrence rate of Dupuytren’s disease and unite current limited fasciectomy practice that varies considerably between surgeons.
Methods
We describe the 3DF principles; raising thin skin flaps (addressing dermal involvement), excising diseased palmar fascia with a 3−5 mm clearance margin (treating highly locally recurrent conditions) and excising the vertical septae of Legueu and Juvara (providing deep clearance, hence addressing all potentially involved pathological tissue). The surgical outcomes between traditional limited fasciectomy (LF) and 3DF are compared.
Results
From the 786 operations included (n=585), postoperative recurrence rates were significantly lower for the 3DF group (2/145, 1.4%) than the LF group (72/641, 11.2%) (P=0.001), and the time to recurrence was significantly longer (5.0±0 years vs. 4.0±0.2 years; P<0.0001). With recurrence excluded, there were no differences between the postoperative complication rates for 3DF (5/145, 3.5%) and LF (41/641, 6.4%) (P=0.4).
Conclusions
Our results suggest that 3DF leads to lower recurrence rates and a longer disease-free period for patients, without increasing complications. 3DF provides a safe, efficacious, common ground surgical approach in the treatment of Dupuytren’s flexion deformity.
INTRODUCTION
Baron Guillaume Dupuytren, a French anatomist and military surgeon, published his description of palmar fascia thickening with subsequent contracture resulting in flexion deformity of one or more digits in 1834 [1]. Although the condition has become eponymous with Dupuytren, Cline’s student Sir Astley Cooper, an English surgeon and anatomist, previously described the same condition in “A treatise on dislocations and fractures of the joints” in 1822 [2]. Since this time, myofibroblast proliferation, transforming growth factor, collagen type III and other proteins have all been implicated in the disease pathogenesis [3].
Surgical fasciectomy remains the most common treatment modality, and several techniques are described in the literature [4-8]. Limited fasciectomy (LF), popularized by Hueston [6], is the most widely used technique; the excision involves removal of the longitudinal fibers of the diseased palmar and diseased digital fascia with a narrow margin of uninvolved aponeurosis. However, surgeons have been unable to agree on a common ground LF definition, for example, some may or may not choose to include the transverse or vertical palmar fascia fibers in their LF excision [5,8,9]. Radical fasciectomy (RF) involves extensive removal of the whole palmar fascia with radical dissections of the palm and any involved fingers [10]. This is not a popular technique as the complications are significantly greater than LF, although the 5-year recurrence rate of 20% to 40% is similar to LF [5,7,8].
Reported postoperative fasciectomy complication rates range from 3.6% to 46% in the literature and include haematoma, neurovascular bundle injury, skin loss, infection, oedema, scar hypertrophy, reflex sympathetic dystrophy and recurrence [4-8,11-16]. Flexion deformity recurrence or disease extension after surgery for Dupuytren’s disease remains one of the most challenging surgical issues. The differentiation between recurrence and extension is controversial, difficult to define and the variety of described surgical methodologies attempt to address such issues [17]. More recent nonsurgical approaches are partly based on the pre-recognition that recurrence is almost inevitable such that surgery is best reserved for more complex cases.
For this study, the definition of recurrence was in keeping with previously published reports; the re-appearance of active disease in a previously operated area, resulting in flexion deformity and requiring release [17-22].
The primary purpose of this study is to describe a common ground surgical approach for the treatment of Dupuytren’s contracture, uniting current LF practice that varies considerably between surgeons, with the aim of excising all potentially diseased tissue leading to a reduced recurrence rate but without the complication rates associated with RF. We have named this approach “3-dimensional fasciectomy (3DF).”
METHODS
The primary aim of this study was to compare postoperative recurrence of LF, with 3DF, a newly defined common ground surgical approach for treating Dupuytren’s disease that is described below. Further aims included subanalysis of epidemiological data and potential clinical severity markers including affected digit, joint and degree of pre-and postoperative joint contracture. The null hypothesis was that no outcome differences would be demonstrated between patients in the LF and 3DF groups.
After registration with and approval from the hospital’s Clinical Governance department (No. 3018), a retrospective review of hardcopy and electronic notes was undertaken for patients who underwent fasciectomy between January 2001 and 2012. Although the two techniques were not offered to patients at presentation, the indication for fasciectomy in all cases was; Flexion deformity resulting in a positive Hueston table top test, with negative impact on the patient’s daily activity [23]. Patients were consented for fasciectomy and the possible risks of the surgery including scar, infection, bleeding, injury to the neurovascular bundle, inability to achieve full extension of the digit, stiffness, recurrence, pulmonary embolism, deep vein thrombosis and the possibility of further surgery.
Patients under the age of 18 years, or those whose hospital notes were not fully traceable, were excluded from the study. Gathered data included; age, sex, profession, hand and digit affected, joint affected, extent of pre- and postoperative flexion deformity, date of initial operation, date of recurrence and presence of diathesis factors. All complications were recorded by both the Hand Therapy and Surgical team, with identical hand therapy protocols (see 3DF approach). Our definition of recurrence was in keeping with previously published reports; the reappearance of active disease in a previously operated area, resulting in flexion deformity and requiring release [17-22]. SPSS software (SPSS Inc., Chicago, UL, USA) was used for statistical analysis. Chi-square was used for non-parametric data analysis and t-test for angles/parametric data analysis where appropriate.
Indications
The indication for 3DF is identical to LF; flexion deformity resulting in a positive Hueston table top test, with negative impact on the patient’s daily activity [23].
Exposure
Under tourniquet control, using a lead hand, Bruner’s (zig-zag) incisions, with Y-V advancement were used for access in our series (Fig. 1). Skoog’s (vertical incision followed by Z-plasty) incisions are reserved for cases where skin shortage is anticipated following fasciectomy. This is usually in long standing cases, advanced flexion deformity cases and in some recurrent cases where skin quality is not ideal and Z-plasty may help skin redistribution.
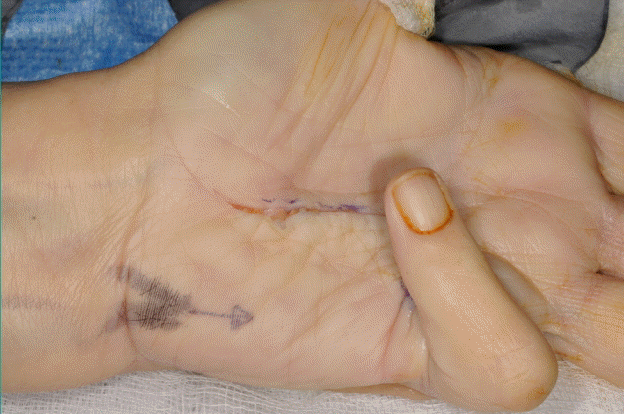
Planning incisions
Under tourniquet control, using a lead hand, Bruner’s incisions, with Y-V advancement were used for access.
Relatively thin flaps are raised at intra-dermal or just subdermal levels, almost the thickness of a full thickness skin graft, in recognition that Dupuytren’s can involve or even originate from the dermis, thereby excising all potentially diseased fascia (Fig. 2). The flaps are reliable and should be raised at a 1:1 (width: length) ratio as their vascular supply originates from the dermalsubdermal plexus; such flaps are reliably used in hand reconstruction [24]. Furthermore, flap survival is augmented by the principles of full thickness skin graft healing due to the intradermal/just subdermal plane of elevation. This step of raising thin skin flaps is not focused on in LF techniques, but is used in 3DF in order to excise all potentially disease fascia.
Excision of diseased tissue
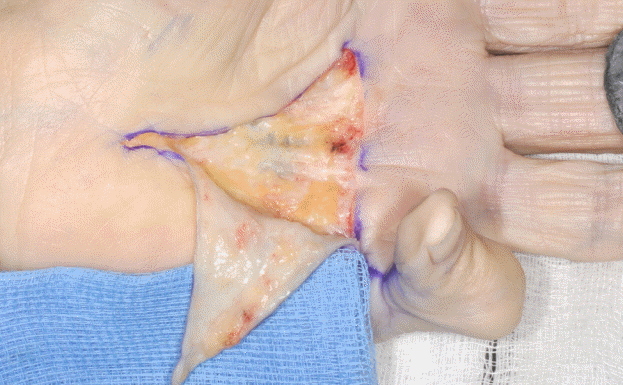
Dupuytren’s is prone to a high local recurrence and/or progression rate. In recognition of this fact, the involved palmar fascia (aponeurosis) fibers, identified through the relatively “greyishdull” appearance of affected tissue, are excised with a 3−5 mm peripheral clearance margin where possible to clear this potentially diseased fascia (Fig. 3). This is the same principle adopted to prevent recurrence in skin lesions with potentially high local recurrence rates or those that are locally malignant such as basal cell carcinomas. This is in contrast to LF, where traditionally only a narrow margin of uninvolved tissue is excised. It also overcomes the possible existence of a “transitional zone” that has a higher propensity for disease progression. This clearance margin is also in contrast to RF techniques where extensive removal of the whole palmar fascia occurs.
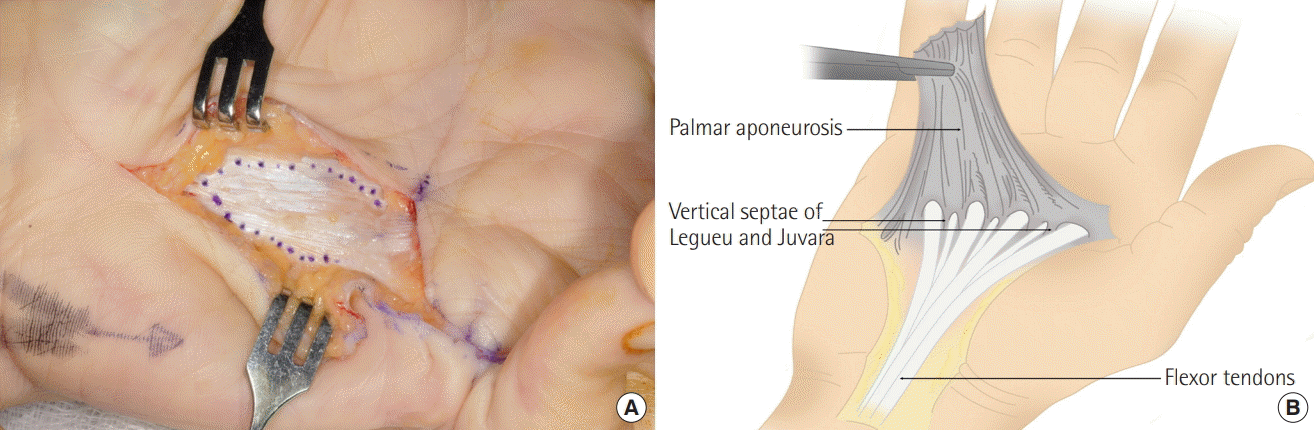
Excision of diseased tissue with a 3–5 mm margin
(A) The involved palmar fascia (aponeurosis) fibers are excised with a 3−5 mm peripheral clearance margin where possible to clear this potentially diseased fascia. This is in recognition of the fact that Dupuytren’s is prone to a high local recurrence and or progression rate. (B) The palmer fascia (aponeurosis) is shown with the underlying septae of Legueu and Juvara. Only the affected fascia is excised with a 3-5 mm clearance margin and the dissection continues to excise the septae of Legueu and Juvara.
Diseased tissue is carefully excised in a proximal to distal fashion, including purposeful routine total excision of all the septae of Legueu and Juvara (vertical fibers) to provide a deep clearance margin, hence addressing all potentially involved pathological tissue (Fig. 4). This step is the fundamental difference to LF, where traditionally only longitudinal fibers are excised, and is what makes the technique “3-dimensional” as it dissects fibers in the longitudinal, vertical and transverse plane. The surgery extends into the digit, whenever there is Dupuytren’s involvement here, in order to achieve complete or near-total correction (Fig. 5).
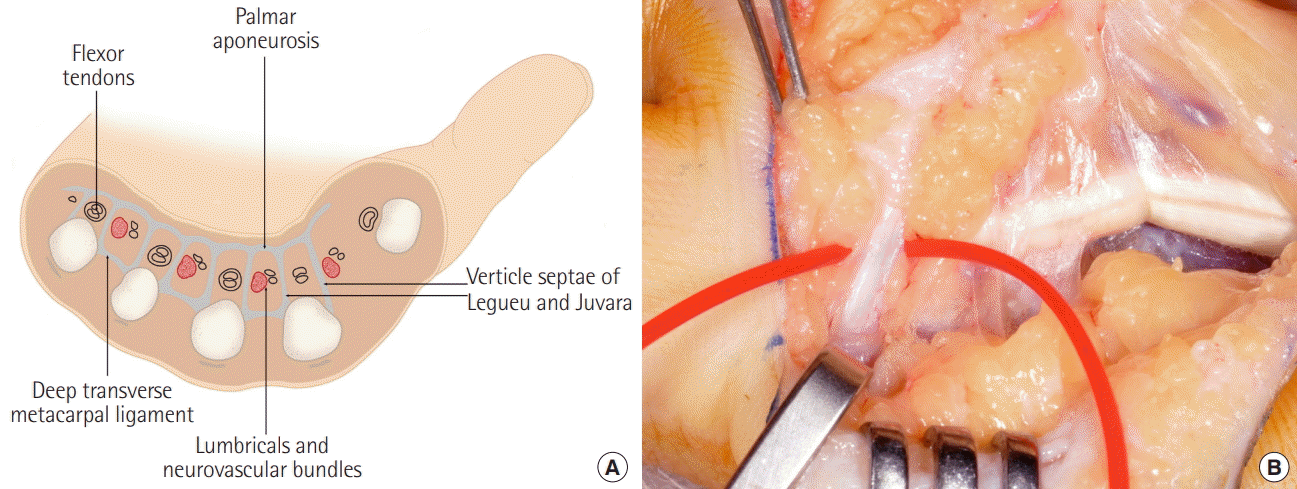
Excision of the vertical septae of Legueu and Juvara
(A) The 8 vertical septae of Legueu and Juvara form 7 compartments through which either the long flexor tendons, or lumbricals and neurovascular bundles run longitudinally. The septae attach distally to the metacarpals, transverse metacarpal ligament and interosseous fascia. (B) Diseased tissue is carefully excised in a proximal to distal fashion, including purposeful routine excision of all the septae of Legueu and Juvara to provide a deep clearance margin. This step is the fundamental difference to limited fasciectomy and is what makes the technique 3-dimensional.
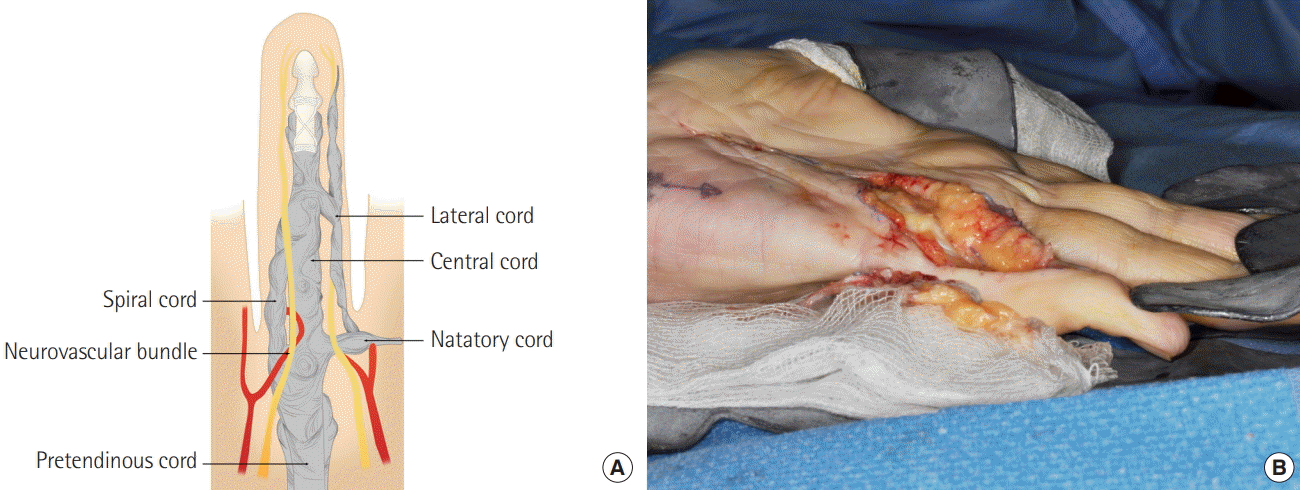
Digital extension of 3-dimensional fasciectomy
(A) The central cord runs longitudinally in the middle of the proximal phalanx, attaching distally to the middle phalanx. The lateral cord runs between the neurovascular bundle and skin, to which it is intimately adherent. The spiral cord is also intimately related to the neurovascular bundles. (B) The surgery extends into the digit, whenever there is Dupuytren’s involvement here, in order to achieve complete or near-total correction.
Closure
The skin is sutured with interrupted 5-0 prolene. A volar splint is applied to maintain intra-operative extension.
Rehabilitation
Standardized rehabilitation, in the form of active and passive mobilisation, is commenced within 48 hours, with progressive splints until ready for discharge from hand therapy.
RESULTS
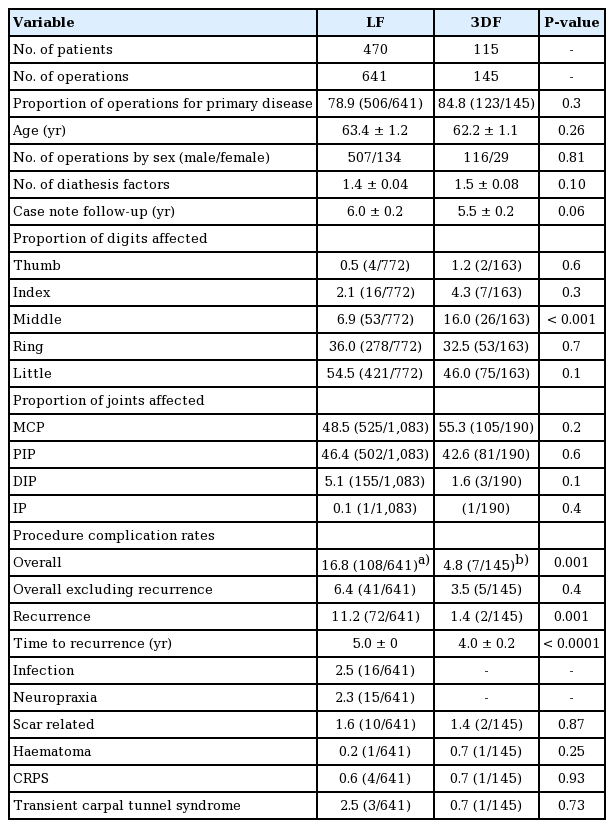
A total of 786 operations, involving 935 digits and 1,273 joints, were performed for 585 patients out of 629 patients whose notes were fully traceable. There were 641 LF (n=470) and 145 3DF (n=115) operations performed, with some patients having had operations using both techniques. Both groups were similar as they were matched for proportion of operations undertaken for primary disease, age, sex, number of diathesis factors, case note follow-up period, proportion of digits and joints affected and extent of preoperative joint flexion deformity; further details are listed (Tables 1 and 2). Patients were not assigned to any surgical method based on severity or difference in disease, rather, the technique was adopted according to the surgeon’s preference as described in the operation notes.
The overall postoperative complication rates were significantly lower for the 3DF group (7/145, 4.8%) than the LF group (108/641, 16.8%) (P=0.001) (Table 1). However, with recurrence excluded, there were no differences between the postoperative complication rates for 3DF (5/145, 3.5%) and LF (41/641, 6.4%) (P=0.4); further complications are listed (Table 1). Postoperative recurrence rates were significantly lower for the 3DF group (2/145, 1.4%) than the LF group (72/641, 11.2%) (P=0.001), and the time to recurrence was significantly longer (5.0±0 years vs. 4.0±0.2 years; P<0.0001) (Table 1).
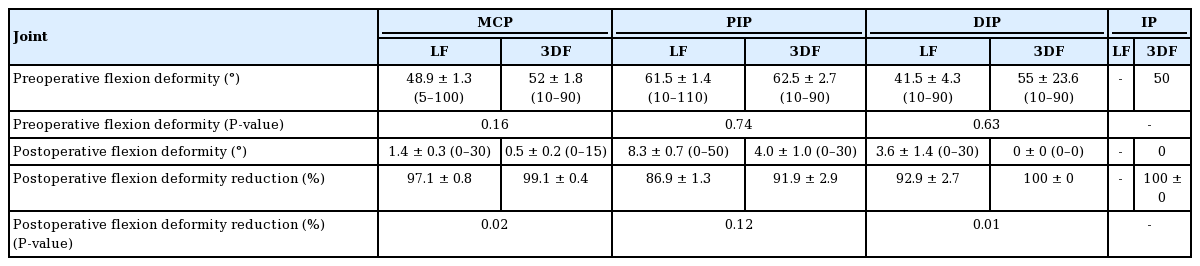
Good postoperative flexion deformity reduction was achieved for all joints regardless of whether LF or 3DF were performed (Table 2). Of interest, there was a significantly greater postoperative reduction in flexion deformity at the metacarpophalangeal (MCP) (99.1%±0.4% vs. 97.1%±0.8%; P=0.02) and distal interphalangeal (100±0% vs. 92.9±2.7%; P=0.01) joints for patients in the 3DF group versus the LF group, however no differences were demonstrated at the proximal interphalangeal joint (Table 2).
DISCUSSION
The principles of 3DF provide a common ground surgical approach for releasing Dupuytren’s flexion deformity (Figs. 1-5); 3DF unites highly variable limited fasciectomy practice between surgeons who, for example, may or may not choose to include the transverse or vertical palmar fascia fibers in their LF excision and take a wider margin of potentially involved fascia. Within the framework of a large retrospective study of two well matched study groups, the presented data (Tables 1 and 2) suggest a lower disease recurrence rate and longer time to recurrence, versus LF, when applying the 3DF principles that address potential contributors to high postoperative recurrence rates in Dupuytren’s disease; raising thin skin flaps (in recognition of the dermal involvement in Dupuytren’s disease), excising diseased palmar fascia with a 3–5 mm peripheral margin where possible (the principle of treating locally recurrent disease), excising the vertical septae of Legueu and Juvara (providing a deep clearance margin, hence addressing all potentially involved pathological tissue) and surgical extension into the digit whenever there is involvement (Figs. 1-5).
Dupuytren’s recurrence is thought to arise from non-excised diseased tissue which may come from under the skin flaps or remaining diseased palmer/digital tissue that was not excised intra-operatively. Therefore, by performing the key features of the 3DF technique, as described, which aims to remove both diseased and potentially diseased fascia, the chance of recurrence should be reduced, as reflected by this retrospective study.
The definition of recurrence in this study was in keeping with previously published reports; the re-appearance of active disease in a previously operated area, resulting in flexion deformity and requiring release [17-22]. Reported postoperative complication rates for Dupuytren’s fasciectomy procedures vary in the literature between approximately 3.6% to 46%, figures in keeping with those found in this study [4-8,11-16]. Of note, both patients who experienced recurrence after 3DF were high risk; one was referred to our department having undergone two previous LF procedures and with four diathesis factors present (male, onset <50 years, bilateral and ectopic disease), the other patient was referred having undergone one previous LF procedure and with four diathesis factors (male, onset <50 years, positive family history and bilateral disease). Despite this, both patients experienced recurrence significantly later than those in the LF group by one year, hence providing further indication of the potential benefit that 3DF may offer.
The robust nature of the thinly raised skin flaps are firstly due to dimensions that are in keeping within the upper limit of random pattern flap principles (3:1, width:length) by utilizing a more reliable 1:1 (width:length) ratio, with vascular supply originating from the dermal/subdermal plexus (Fig. 2) [24]. Secondly, flap survival is augmented by the principles of full thickness skin graft healing due to the intra-dermal/just subdermal plane of elevation. While there were no cases of flap loss with 3DF in this study, if patients were to experience this, healing would still ensue with dressings according to the principles of the McCash open palm technique [25]. The 3–5 mm excision peripheral palmar disease clearance margin where possible may overcome the possible existence of a ‘transition zone’ that has a higher propensity for disease progression (the principle of treating highly locally recurrent disease) (Fig. 3); excising all of the vertical septae of Legueu and Juvara effectively provides a deep clearance margin thereby addressing all potentially involved pathological tissue (Fig. 4). The surgical dissection extends into the digit whenever there is Dupuytren’s involvement which acknowledges that disease mostly starts in the palm with patients developing digit flexion deformity after MCP flexion deformity.
We acknowledge that individual surgical techniques may vary between surgeons and due to this recognize the future benefit of a prospective randomized controlled trial. Despite this, our results strongly suggest that that the principles of 3DF, when utilized in combination, are equally as safe as other release methodologies and lead to lower recurrence rates and a longer disease-free period for patients. The principles of 3DF provide a safe, efficacious, common ground surgical approach for hand surgeons considering fasciectomy for patients with Dupuytren’s flexion deformity.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Clinical Governance department of the Countess of Chester Hospital (No. 3018) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and the use of their images.