METHODS
Approval was obtained for this study from the Animal Ethics Committee of Malaysia Science University, and it was conducted according to the rules and requirements for an animal laboratory study as determined by the committee. Eighteen albino New Zealand male rabbits with an average weight of 4,000 g each were used. The first two rabbits were dissected to familiarize the researchers with their anatomy. The remaining 16 rabbits were divided into 4 groups comprising 4 rabbits each. Each of these groups was then subdivided into 2 subgroups comprising 2 rabbits in each group, with one subgroup undergoing ischemic preconditioning.
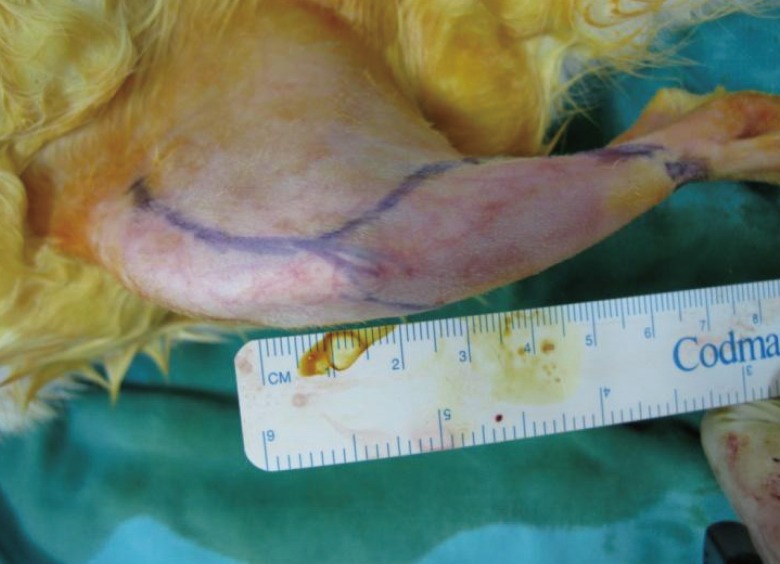
Each rabbit underwent surgery through a lateral incision approach to one hindlimb to locate the distal femur (
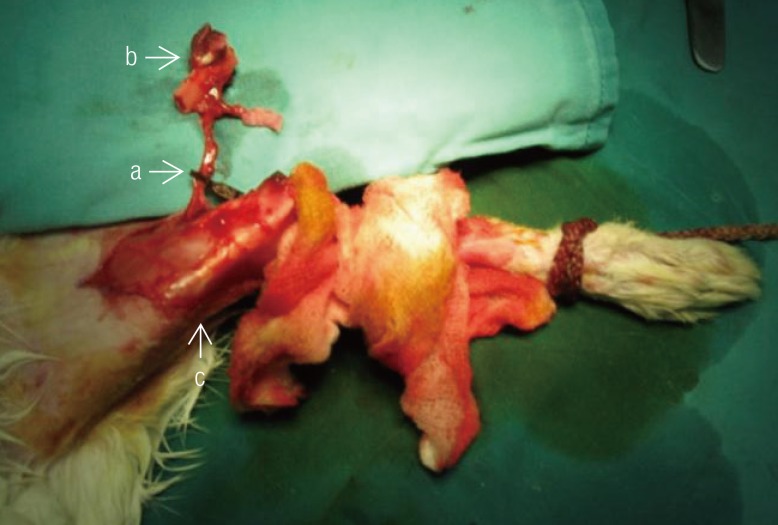
Fig. 1). Following this, the saphenous artery, which is the vascular pedicle to the bone graft, was dissected up to the femoral vessel. Osteotomy was then performed on the distal portion of the femur based on this vascular pedicle and the vascularized bone graft was elevated together with an overlying skin paddle (
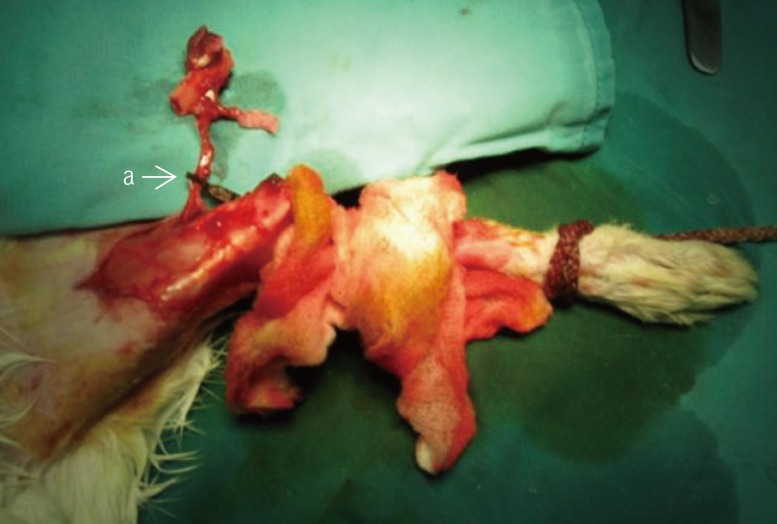
Fig. 2). The vascular pedicle was clamped for the designated ischemic time before the blood flow was re-established and the bone graft replaced into the original site and fixed using K-wires (
Fig. 3). Skin paddle size was standardized at 2 cm
2 and the bone graft length at 2 cm. Only 1 K-wire was used for each limb. Finally, the skin paddle was resutured back to the surrounding skin. Out of the total of 18 rabbits, 2 rabbits were earlier dissected to become familiar with the anatomical findings. The remaining 16 rabbits were divided into 4 groups comprising four rabbits each. The rabbits in group 1 had an intraoperative ischemic time of 2 hours; group 2, 6 hours; group 3, 14 hours; and group 4, 18 hours. Immediately following this, two of the rabbits of each subgroup underwent ischemic preconditioning. There was no delay in time prior to subjecting the rabbits to ischemic preconditioning. The technique of ischemic preconditioning involved applying a vascular clamp on the pedicle for 3 cycles of 10 minutes each, with a release of clamping of 10 minutes between each cycle.
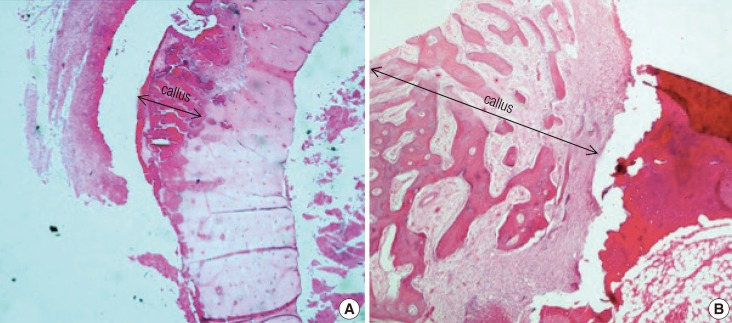
The percentage of skin flap necrosis was compared among the experimental group by using planimetry. These assessments were performed on day 2, day 4, and day 6 following the procedure. All of the findings were documented in a standard form. Macroscopic assessment of bone grafts was later performed by two blinded assessors on the sixth week after they were harvested prior to sectioning for histological analysis. The assessment for the bone grafts included the gross appearance of the proximal and distal host-graft junctions in terms of bony union as well as the viability of the bone graft shafts themselves. Bony union was defined by an immobile host-graft junction with the presence of a bridging callus. The bone grafts were defined as viable based on the presence of healthy tissues and absence of necrotic segments.
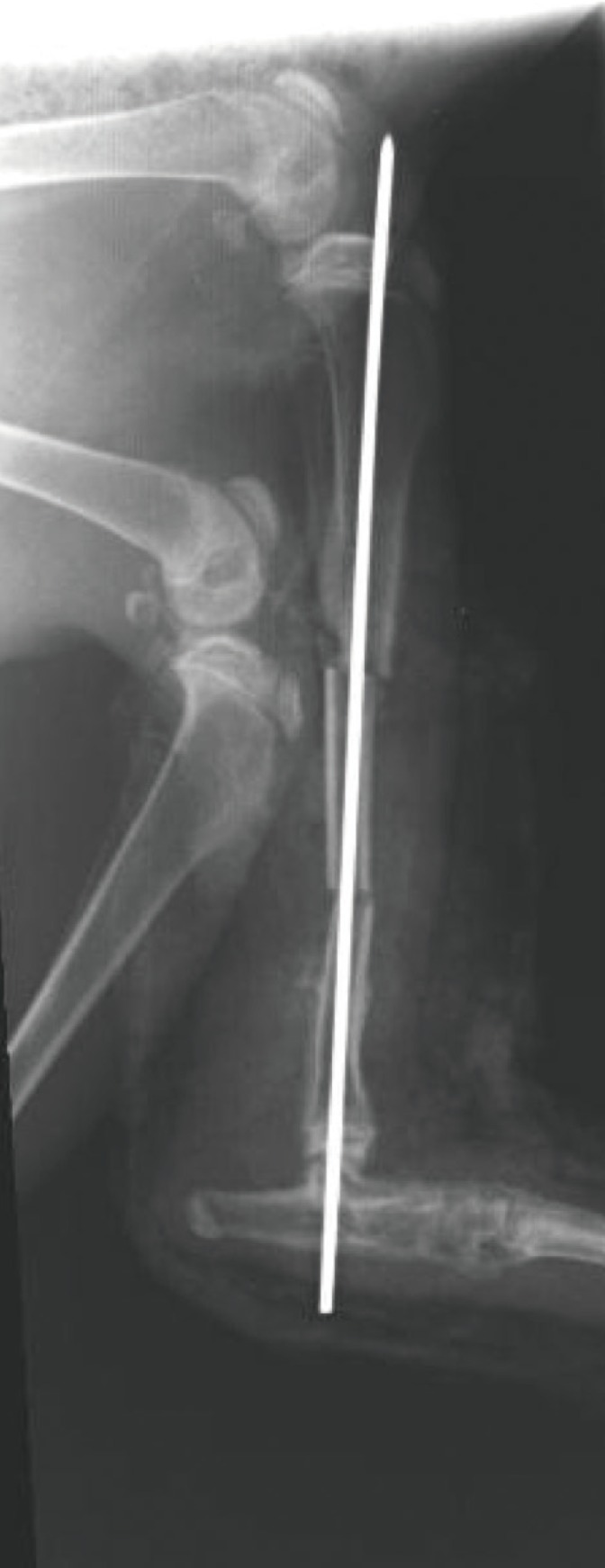
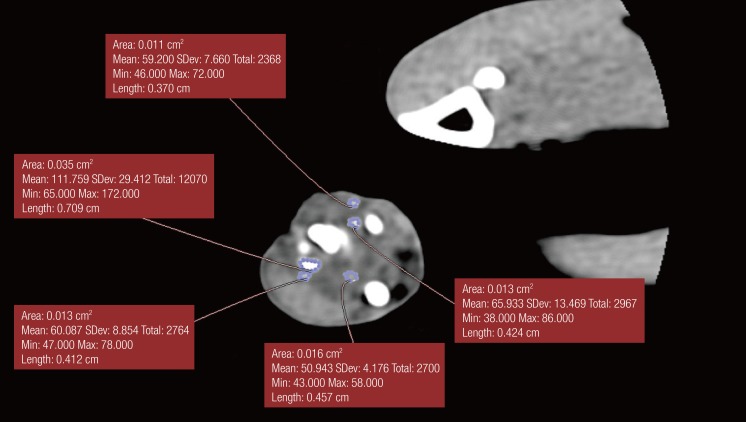
Each animal underwent radiological evaluation, just after, 2 weeks after, 4 weeks after, and 6 weeks after the procedure when significant bone healing was anticipated (
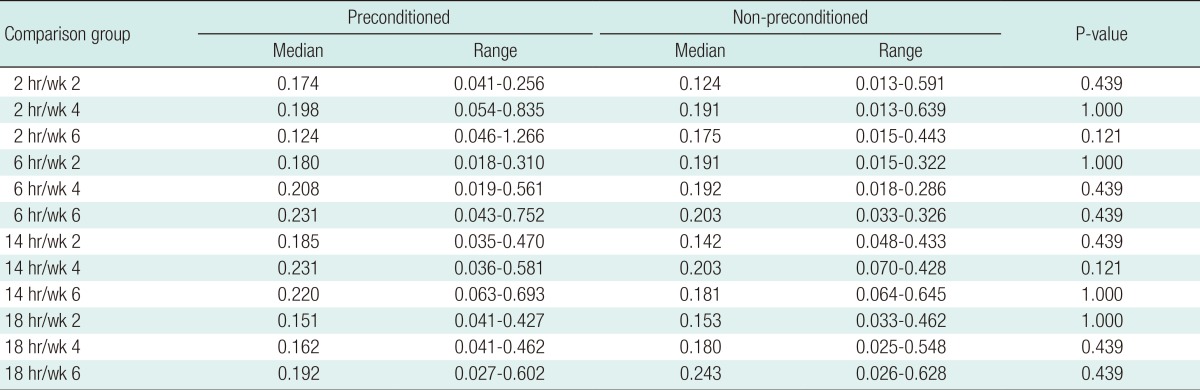
Fig. 4). A modification of the Sao Paolo classification system was used for the interpretation of the plain radiograph images. The computed tomography (CT) scans were analyzed using the software OsiriX ver. 3.5.1 64-bit. The soft tissue window was chosen, as it showed better delineation of the callus formed. A free hand technique was chosen to outline the area of the callus for each slice, and then the mean reading of the callus area was calculated. Statistical calculation using the Mann-Whitney test was then used to determine the significance of differences between the study and control groups.
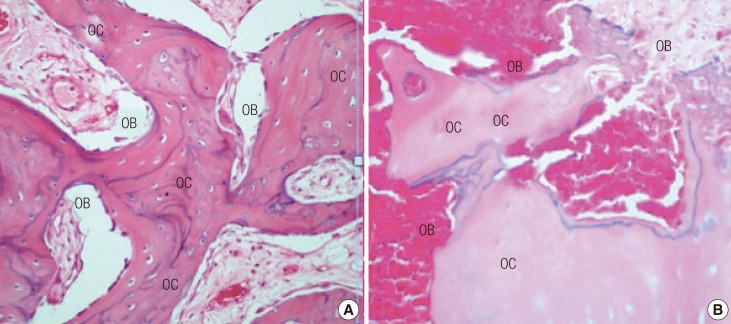
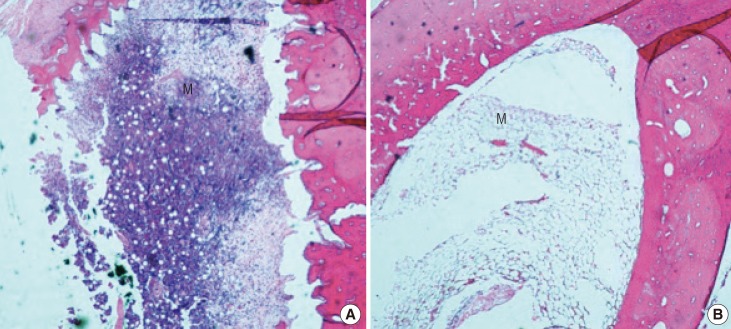
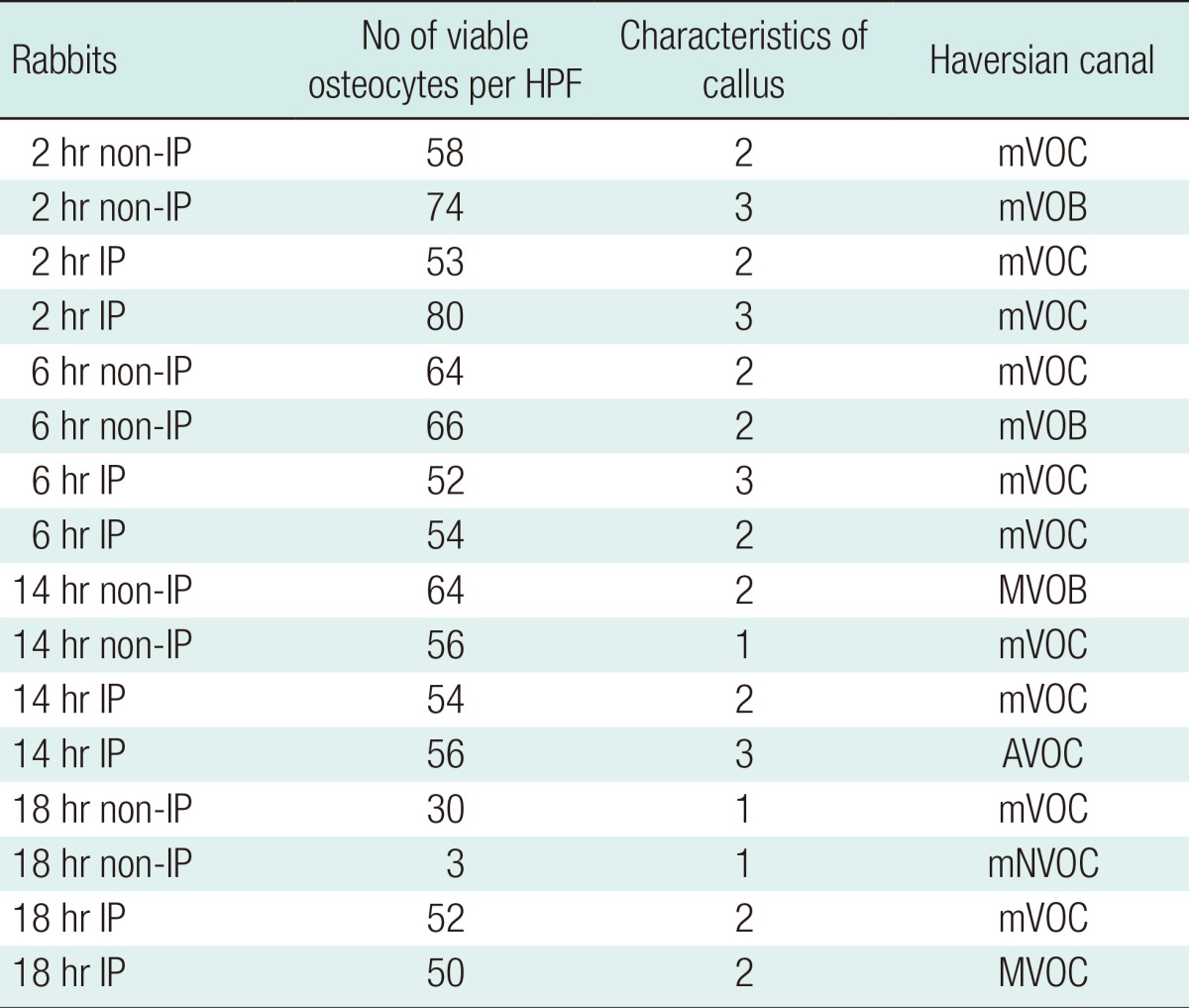
All of the rabbits were sacrificed at the end of the sixth week. Decalcification was achieved by immersing the entire tibia shafts in 5% nitric acid. Vertical sections from the proximal host-graft junctions were made. These were stained with hematoxylin and eosin. Assessment of each slide was performed under microscope to determine the average number of viable osteocytes per 10 high power fields (HPF); whether the callus was minimal, moderate, or abundant; the appearance of the Haversian system; and whether the marrow appeared viable or non-viable (
Figs. 5-
8).
DISCUSSION
The clinical usage of a free vascularized bone graft during the reconstruction of complex skeletal defects often requires a prolonged intraoperative ischemic time. This time is required during the harvesting of the bone graft and preparation of the recipient site, as well as the duration of the microvascular anastomosis [
8]. Even though osteocytes are known to have a high level of tolerance to ischemic insults, these cells, as with any living cells, are still susceptible to the grievous results of prolonged intraoperative deprivation of the blood supply [
9]. One of the methods that has been studied extensively to enhance the viability of flap tissues is ischemic preconditioning. Ischemic preconditioning effectively belongs on a spectrum with ischemic reperfusion injury on the other extreme end.
One well-documented event during ischemic reperfusion injury is the attachment of platelets and leukocytes to vascular endothelial cells. Leukocytes also migrate into the extravascular space, where they release oxygen radicals, peroxynitrite, and enzymes that can damage or degrade tissues, including the heat shock proteins. Intravascular deposition of fibrin can also occur during a ischemic reperfusion injury [
10]. Other than this, there will be edema, causing an increase in the passage of macromolecules and fluid from the vascular to the extravascular compartment [
9].
Experimentally, the heat shock response has been demonstrated to have a positive effect on flap survival by protecting tissue from ischemia or ischemia-reperfusion injury [
11]. These proteins are a superfamily of highly conserved intracellular proteins that protect cells and whole organisms from various stresses. The resulting induction of the heat shock proteins provides the cells with cross-protection from subsequent injuries. Apart from the effects of heat shock proteins, ischemic preconditioning also acts via the action of adenosine triphosphate (ATP)-sensitive potassium channels, arteriolar dilatation, iron regulatory protein, and the release of substances after prolonged ischemia such as adenosine, bradykinin, opioids, and oxygen radicals [
2,
12-
15].
As this was a pilot study using an animal model, the sample size was small in number. Berggren et al. [
8] have shown the effect of prolonged ischemic time on osteocytes and osteoblasts in vascularized bone grafts anastomosed through microsurgical techniques. The ischemic time varied from 90 minutes to 48 hours. His results showed that a vascularized bone graft could survive an ischemic time of up to 25 hours. We have chosen an intraoperative ischemic time of between 2 hours and 18 hours as this, in our opinion, closely resembles the operative times in clinical settings.
The subsequent pedicle clamping technique was used instead of microsurgical anastomosis for several reasons. Even though microsurgical anastomosis would more closely resemble the actual clinical settings of free tissue transfer, there were several confounding factors that would affect the outcome of the procedure. This technique would consume a great deal of operative time to complete in each rabbit, and even after successful completion of the anastomosis, the flap would be exposed to the risk of arterial or venous thrombosis or anastomotic breakdown further requiring flap reexploration [
16]. Early detection of any arterial or venous problem would be very difficult and even if we managed to detect it, emergency flap re-exploration might not be possible to perform for logistical reasons. This will further complicate the procedure and delay the completion of this project. Thus, through clamping of the flap pedicle, we were also able to create a complete cessation of the blood supply to and from the flap, essentially producing a state of ischemia during flap transfer, without all the problems of microvascular anastomosis as mentioned earlier.
In comparing between the preconditioned and non-preconditioned animals, in the 2-hour groups, the areas of skin flap survival were equal for the two groups during all wound inspections. The 2-hour ischemic time was relatively too short to influence the survival of the keratinocytes. In the 6-hour ischemic time groups, better survival was seen in the preconditioned groups. This duration of ischemia may mimic more actual clinical scenarios. This finding correlates with most of the other studies on the effects of ischemic preconditioning on skin flaps [
15].
For the longer ischemic time groups of 14 and 18 hours, it was found that all of the skin flaps in the non-preconditioned rabbits were not viable. However, in the preconditioned group, both rabbits showed small areas of skin flap survival. Zahir et al. [
17] examined the effects of ischemic preconditioning on 220 rats via 100 transverse rectus abdominis myocutaneous flaps and 120 dorsal cutaneous flaps. His results showed that for the skin flap model, statistical significance of the survival area difference was reached at 6, 10, and 14 hours of ischemia.
In rabbits, good union after fracture healing was seen at the sixth postoperative week [
17]. Plain radiograph analysis of the 2 groups was performed using a modified scoring system based on the Sao Paolo classification [
18]. This scoring system was used because of its simplicity in interpreting the results. We have modified it by combining some categories that were difficult to clearly differentiate. Inter-observer variability during the assessment of plain radiographs has been reported to be between 20% and 25% [
19].
The process of bone healing followed the natural process with mostly dense callus tissue seen in the sixth week radiographs. Early callus formation was beginning to be seen in all of the groups after the fourth postoperative week. The findings from the plain radiographs trace the process of fracture healing, with changes starting to be visible from the third to fourth week after fracture. This correlates with the physiological phase of wound healing, when the hard callus stage usually starts [
20]. In this study, at the sixth week, for the 2, 6, and 14 ischemic hour groups, a higher score for dense callus and signs of fracture healing were seen in the preconditioned animals. The difference was greater in the groups with a shorter ischemic period than the 18-hour group. We postulated that in the 18-hour group, the extreme ischemic time resulted in widespread osteocyte death even before preconditioning was commenced. However, future studies to specifically prove this theory are warranted.
In this study, a compounding factor that needed to be taken into consideration was the presence of internal fixation in between the fracture segments. Internal fixation affects the mechanical and biological environment of a fracture, hence affecting the amount of callus form and cortical bridging. Primary healing occurs only when anatomic restoration of the fracture fragments takes place, by rigid internal fixation, and when the stability of fracture reduction is ensured by a substantial decrease in interfragmentary strain. Under these conditions, bone-resorbing cells on one side of the fracture show a tunnelling resorptive response, whereby they re-establish new Haversian systems by providing pathways for the penetration of blood vessels. The amount of callus formation is influenced by the relative stability of the fracture fragments. The more motion there is at a fracture site, the larger a callus is needed to prevent this motion [
21].
The outcome showed an increasing quantity of callus formation from week 2 to week 6 in all of the groups. In comparison between with the animals that underwent ischemic preconditioning, as seen on the CT scans, those without preconditioning appeared to have more callus tissue formation in the subgroups of 2 hours, 6 hours, and 14 hours. However, the non-preconditioned group showed more callus formation in the 18-hour group. Therefore, we postulated that at 18 hours, the initial number of non-viable osteocytes upon completion of the procedure was more than in the other groups; thus, unlike the others, they were not influenced by ischemic preconditioning. However, further studies looking into the histochemical changes after the respective ischemic times will objectively prove this. As for quantitative evaluation, the CT analyses showed good intraobserver and interobserver reproducibililty [
22]. Only 16 rabbits were used, as this was a pilot study. As the data were non-parametric, we chose the Mann-Whitney test for statistical evaluation. This would inflate the type I errors by running so many tests at the same time, even though it may not be necessary to correct type I errors for this exploratory analysis. The results showed no significant difference between the preconditioned and non-preconditioned groups. With a larger sample size, we would expect the result to be significantly different with more favorable outcomes in the ischemic preconditioned groups. We would have the ability to obtain more precise data by limiting errors caused by confounding factors, which are a limiting factor with a smaller sample size. Additional animals in each preconditioning time subgroup would also increase the power for calculation to determine the significance of the statistical results.
The technique of ischemic preconditioning used in this study involved the application of vascular clamps on the flap pedicles for three cycles of 10 minutes each. We based our technique on a study performed by Zahir et al. [
17] that applied the same preconditioning protocol during the transfer of a pedicled rectus abdominis myocutaneous flap in a rat model. The ischemic preconditioning protocol used for this study allowed an even distribution of time for ischemic preconditioning and reperfusion of the flap models. The period of 10 minutes was also deemed adequate to expose the osteocytes to the ischemic insult without creating the unnecessary damage to the cells that an excessive exposure would. This is based on an ischemic preconditioning model of skin flaps by Zahir et al., [
17] as no other studies of ischemic preconditioning on bone grafts have been reported. We did not use a shorter ischemic time, as it might be too brief to create the preconditioning effects to the bone cells.
A myocyte model of ischemic preconditioning has been shown to lead to rapid phosphorylation of potassium-adenosine triphosphate channels, which leads to the cardioprotective effects of K
+ efflux. Ischemic preconditioning has also been shown to result in heat shock-mediated arteriolar dilation associated with an increase in volumetric blood flow in individual capillaries. The increased capillary volume flow is caused by capillary dilation rather than increased red blood cell velocity. Katori et al. [
23] demonstrated that ischemic preconditioning also enhanced the action of heat shock proten HSP-32. Competitive inhibition of HSP-32 function completely abrogated the protection from necrosis. In addition to this, previous studies on renal and myocardial reperfusion and re-oxygenation have indicated that HSP-32 attenuates apoptotic cell death. Hutter et al. [
24] demonstrated that in rabbits, the brief period of preconditioning ischemia and reperfusion releases adenosine, bradykinin, opioids, and oxygen radicals. The combined effect of the release of these substances on G proteins and the cell's phospholipases induces the translocation and activation of the ε isozyme of protein kinase C. Protein kinase C appears to be the first element of a complex kinase cascade that is activated during the prolonged ischemia in preconditioned hearts. Current evidence indicates that this cascade contains at least one tyrosine kinase and ultimately leads to the activation of p38 mitogen-activated protein kinase. This substance is one of the proteins that controls actin filament polymerization, and, therefore, affects the integrity of the cytoskeleton [
10].