INTRODUCTION
The poor prognosis of cutaneous malignant melanoma remains an important issue, and many epidemiological studies have been conducted to identify factors that influence the survival and prognosis of patients with malignant melanoma [
1,
2,
3]. In particular, the possibility of the iatrogenic spread of tumor cells and the resulting metastasis caused by the incisional biopsy of melanomas remains controversial.
The aim of this retrospective study was to evaluate the effect of the type of biopsy on the metastatic rate and the prognosis of cutaneous melanoma in 81 patients who were managed at our hospital during a 25-year period.
METHODS
The medical records of 109 patients with cutaneous malignant melanoma who were managed at Tokushima University Hospital from 1983 to 2007 were reviewed. After excluding 28 cases with either stage 0 tumors (malignant melanoma
in situ) or incomplete data, 81 cases were analyzed in detail with respect to patient age, sex, tumor site, Union for International Cancer Control (UICC) TNM stage [
4] at diagnosis, and prognosis.
The patients were divided into three groups (incisional, excisional, and no biopsy) according to the presence/absence of biopsy and the type of biopsy performed. The incisional biopsy group included punch biopsies. Of the 20 incisional biopsies, 19 were single biopsies, while 1 was taken from 3 separate points. The residual tumor after an incisional biopsy was resected with a margin of 3 to 5 cm, and the defect was covered with a skin graft or a skin flap within 2 to 3 weeks. The defect after an excisional biopsy was temporarily covered with artificial dermis. In the "no biopsy" group, a diagnosis was made on the basis of the gross appearance of the lesion and/or dermoscopic findings, and a biopsy was not performed. Distal metastasis and local recurrence were determined by physical examination and either computed tomography or positron emission tomography. Excluding two cases with absent staging data, the five-year survival rate of melanoma patients according to the clinical stage was calculated. The clinical features of patients in the three biopsy groups were analyzed. The five-year survival and five-year disease-free survival rates were calculated for each group.
The survival rates were determined and compared using the Kaplan-Meier method, log-rank test, and Cox proportional hazard regression, using StatView ver. 5.0 for Windows software (SAS Institute Japan Ltd., Tokyo, Japan). Other statistical analyses were performed using Pearson's chi-squared test and analysis of variance, using the SPSS ver. 21 (Japan IBM, Tokyo, Japan). The statistical significance level was set at P<0.05.
RESULTS
Of the 81 patients with malignant melanoma, 37 (45.7%) were male and 44 (54.3%) were female. Their ages ranged from 19 to 93 years (mean, 61.6 years). The most common tumor site was the lower extremity (54.3%) followed by the trunk (16%) (
Table 1). The number and the percentage of patients according to the UICC stage are given in
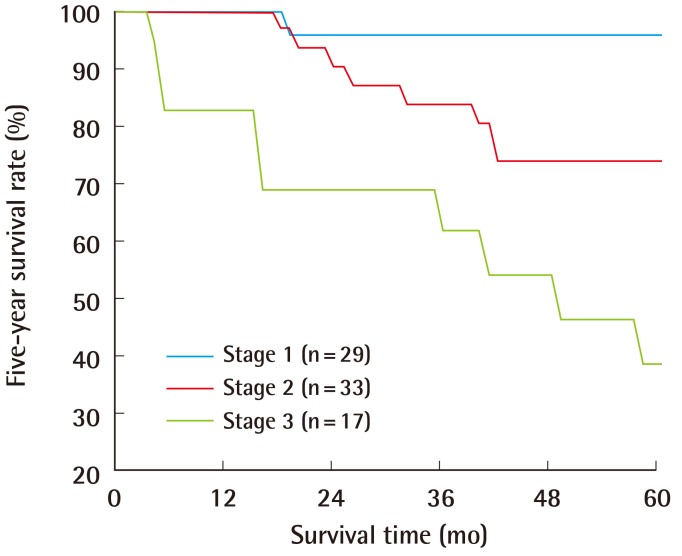
Table 2. Stage I, II, and III tumors comprised 35.8%, 40.7%, and 21% of all cases, respectively. Of all cases, 23.5% and 12.3% were stage IA and IB cases, respectively, while 16%, 16%, and 8.6% were stage IIA, IIB, and IIC cases, respectively. Of all cases, 3.7%, 11.1%, and 6.2% were stage IIIA, IIIB, and IIIC cases, respectively. The five-year survival rates were 95.7%, 73.8%, and 39.2% for stage I, II, and III tumors, respectively (P=0.0003) (
Fig. 1).
The incisional, excisional, and no biopsy groups contained 20, 31, and 30 patients, respectively (
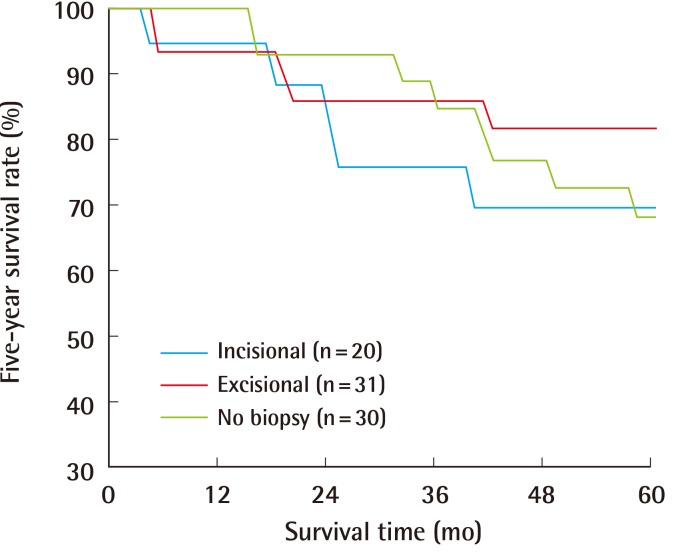
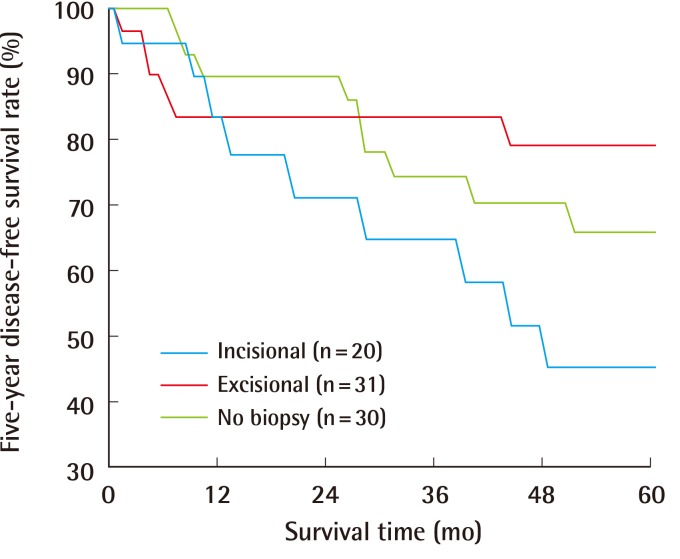
Table 1). No significant differences among the three groups existed with respect to patient age (P=0.139), sex (P=0.442), or tumor site (P=0.170). The mean Breslow thicknesses were 2.94 mm, 2.29 mm, and 3.59 mm in the incisional, excisional, and no biopsy groups, respectively. The number of patients with ulceration was 9, 7, and 14 in the incisional, excisional, and no biopsy groups, respectively. The presence of lymph node metastasis was diagnosed by either sentinel lymph node biopsy in 12 cases or lymph node dissection in 45 cases, while neither was performed in the remaining 24 cases. Of the 57 cases examined for lymph node metastasis, metastasis was found in 15 cases. No significant differences in the mean Breslow thickness, ulceration, and lymph node metastasis were observed among the groups (P=0.092, 0.105, and 0.115, respectively). The type or absence of biopsy did not significantly affect the five-year survival rate (P=0.659) and the five-year disease-free survival rate (P=0.153) (
Figs. 2,
3). The median duration of follow-up was 61 months (range, 3-241 months). The hazard ratios for five-year survival in the incisional and excisional biopsy groups were 1.11 (95% CI, 0.36-3.39) and 0.65 (95% CI, 0.21-2.00), respectively. The hazard ratios for five-year disease-free survival in the incisional and excisional groups were 1.80 (95% CI, 0.72-4.55) and 0.69 (95% CI, 0.25-1.95), respectively.
DISCUSSION
Ishihara et al. [
1] investigated 2,065 melanoma patients who were registered with the Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society during 1987 to 2001. The characteristics of patients in our study were similar to those in the study conducted by Ishihara et al. [
1] with respect to age, sex, tumor site, and clinical stage. They reported that the five-year survival rates of patients with stage I, II, and III disease were 90%, 72%, and 54%, respectively, which were similar to the survival rates obtained in our study.
Dermoscopy is helpful for the diagnosis of cutaneous malignant melanoma. However, a pathologic examination of a biopsy of the lesion is required for a definitive diagnosis and determination of the tumor thickness. The influence of the type of biopsy on the patient outcome in melanoma, specifically, whether an incisional biopsy causes local or systemic tumor dissemination, has been debated [
5,
6,
7,
8
9]. Rampen et al. [
5] reported a decreased survival rate in a group of 14 patients who had incisional biopsies compared with that of 62 patients who had excisional biopsies. In a study on head and neck melanomas, Austin et al. [
6] reported a decreased survival rate among patients who had incisional, shave, or needle aspiration biopsies compared with those who had excisional biopsies, although a significant age difference was noted among the patient groups. Lederman and Sober [
7] reported no difference in survival between 119 patients with stage I melanoma who had incisional biopsies and 353 who had excisional biopsies. Lees and Briggs [
8] reported that the type of biopsy had no impact on local recurrence or survival in 1,086 cases of stage I melanoma. Bong et al. [
9] reported that the type of biopsy had no significant effect on recurrence or melanoma-related death rates in 265 and 496 melanoma cases that underwent incisional and excisional biopsies, respectively, after controlling for patient age, sex, tumor site, and Breslow thickness. The present study demonstrated that there were no significant differences in the survival or metastatic rate among the three biopsy groups. Despite the small number of patients in each group, no significant differences in the clinicopathological features were observed among the groups. Because the biopsy method reportedly affects the survival rate of patients with head and neck melanoma [
6], the influence of biopsy type on survival might differ according to the tumor location or other factors. More studies with a large patient population and longer follow-up periods are necessary to explore this possibility.
An incisional biopsy can be performed under local anesthesia in an outpatient clinic and is sufficient for diagnosing melanoma. However, an incisional biopsy provides a small piece of tissue, so it is possible to underestimate the extent of tumor invasion. Information on the depth of tumor invasion is essential in guiding surgical treatment including the excision margin and lymph node dissection. Tumor depth can be determined from an excisional biopsy but not from an incisional biopsy. An excisional biopsy can also be performed under local anesthesia for small melanomas. However, general anesthesia is required in the case of large melanomas, and the post-biopsy defect requires wound coverage for pain and bleeding. Artificial dermis is an excellent option for covering the wound after an excisional biopsy. Excisional biopsies of large melanomas were difficult prior to the availability of artificial dermis for wound coverage. In the present study, some patients in the no biopsy group underwent an excisional biopsy because wound coverage using artificial dermis was not available at the time. The use of incisional biopsies has generally been discouraged because it might affect patient outcome. All patients in the no biopsy group were diagnosed with melanoma based on typical clinical and/or dermoscopy findings.
The mechanism by which incisional biopsies increase the risk of melanoma metastasis is unknown. Some researchers postulate that the release of fibroblast growth factors during wound healing after a diagnostic incisional biopsy may promote melanoma progression. Hofer et al. [
10] found that fewer melanoma cells were needed to induce melanomas in rats with surgical wounds and that the injection of early wound fluid increased the tumor size.
Our results are consistent with the opinion that the biopsy technique does not affect the prognosis of melanoma patients. The use of incisional biopsies is generally discouraged because accurate measurements of tumor thickness are not possible. As the type of biopsy does not affect the melanoma recurrence rate or prognosis, we believe that an incisional biopsy can be selected over an excisional biopsy if the clinical diagnosis of melanoma is uncertain. An excisional biopsy (followed by wound coverage with artificial dermis) is useful in guiding subsequent treatment because it reveals the thickness of the tumor.